Introduction. Poor adherence to treatment for various chronic diseases is a frequent phenomenon. Current guidelines for the treatment of chronic hepatitis B (HBV) and hepatitis C (HCV) recommend optimal adherence, since it has been suggested that poor adherence is associated with an increased risk of virological failure. We aimed to give an overview of studies exploring adherence to combination treatment (PEG-interferon plus ribavirin) for HCV and nucleos(t)ide analogues for HBV.
Material and methods. A systematic review was conducted using the databases PubMed, Embase, Cochrane Library and Web of Knowledge. Search terms included “adherence” or “compliance” combined with “hepatitis B”, “hepatitis C” or “viral hepatitis”.
Results. The final selection included 19 studies (13 HCV, 6 HBV). Large differences in patient numbers and adherence assessment methods were found between the various studies. For HCV mean adherence varied from 27 to 97%, whereas the proportion of patients with ≥ 80% adherence varied from 27 to 96%. Mean adherence reported in HBV studies ranged from 81 to 99%, with 66 to 92% of patients being 100% adherent. For both HCV and HBV studies, the highest adherence rates were reported in studies using self-report whereas lower adherence rates were reported in studies using pharmacy claims. Poor adherence to treatment was associated with an increased risk of virological failure.
Conclusion. Non-adherence to treatment in chronic viral hepatitis is not a frequent phenomenon. However, given the increased risk of virological failure in poorly adherent patients, clinicians should routinely address adherence issues in all patients treated for chronic viral hepatitis.
Chronic viral hepatitis is a worldwide problem with 170 million patients chronically infected with hepatitis C virus (HCV) and 350 million persons with chronic hepatitis B virus (HBV).1,2 Chronic viral hepatitis can lead to liver cirrhosis, decompensated liver disease and hepatocellular carcinoma (HCC). It has been estimated that 16% of chronic HCV and 8–17% of HBV patients develop liver cirrhosis within 20 years. Approximately 10% of cirrhotic patients will develop HCC within 5 years.3,4 The ultimate goal of treatment is therefore to prevent these complications by achieving adequate viral suppression. In the past decade, the recommended treatment for HCV consisted of pegylated interferon (PEG-IFN) and ribavirin (RBV) for all HCV genotypes.5 The protease inhibitors boceprevir and telaprevir have recently been approved for HCV genotype 1.6,7 Five nucleos(t)ide analogues (NUCs) are currently approved for the treatment of chronic HBV: lamivudine (LAM), adefovir (ADV), entecavir (ETV), tenofovir disoproxil (TDF) and telbivudine (TBV).8 In addition, PEG-IFN can also be used to treat chronic HBV.8
Current guidelines emphasize that optimal adherence to antiviral medication is needed to achieve the best results.5,9 In clinical trials, adherence rates for other chronic diseases range from 43-78% in general, with a radical drop after 6 months of treatment.10 Furthermore, an association between adherence and response to treatment has been reported for various chronic diseases, including hypertension11 and HIV.12 For example, ≥ 95% adherence to protease inhibitors in HIV patients has been associated with non-detectable viral loads.12 At this moment, there is no widely accepted standard of what can be considered good adherence for treatment of chronic viral hepatitis. The 80/80/80 rule is generally used in HCV combination therapy,9 but this is based on dose reductions by physicians due to side effects rather than missed doses by the patient.13
Adherence to therapy can be measured by several methods, including patient self-reports, pill counts, prescription refill rates, electronic medication monitors and measurement of drug levels in blood.10 All methods have advantages and disadvantages and none is considered to be the gold standard.10,14,15 The two most commonly used methods are patient self-reports and pharmacy claims data. Self-report can either be done by questionnaires, oral self-reports or visual analogue scales (VAS) in which patients are asked to indicate their adherence between 0 and 100% in a diagram.
This systematic review aims to give an overview of studies exploring adherence to combination treatment (PEG-interferon plus ribavirin) for HCV and NUC regimens for HBV. Furthermore, we summarize studies that evaluated predictors of poor adherence and assessed the relation between virologic response and adherence.
Material and MethodsSearch strategyWe performed a systematic literature search in 4 electronic databases: PubMed, Embase, The Cochrane Library and Web of Knowledge, thereby identifying all relevant published articles and abstracts until April 20, 2012. The following search terms were used: “adherence” or “compliance” and their synonyms (in title or abstract) combined with “hepatitis B”, “hepatitis C” or “viral hepatitis” and their synonyms (in title). In addition, reference lists of retrieved articles were manually searched, and reviews were evaluated.
Inclusion and selection of studiesPublished articles and abstracts of randomized controlled trials, prospective cohort studies and retrospective cohort studies were included when the following inclusion criteria were met:
- •
The study population consisted of treated adults with HBV or HCV.
- •
The primary or secondary aim of the study was evaluation of medication adherence.
- •
Medication adherence was defined as the proportion of medicaments taken by the patient as described by the physician (studies that defined adherence as dose reduction and/or early treatment discontinuation were excluded).
- •
Publication was in English or Dutch.
If multiple publications reported on the same cohort, the study with the longest follow-up was included. Reviews, case reports, meta-analyses, pediatric, animal or laboratory studies were excluded. Two independent reviewers screened all studies. Publications judged relevant based on title and abstract were further evaluated as full article. Disagreement was resolved by discussion.
OutcomesThe primary outcome of this review was medication adherence. Predictors of poor adherence and correlation between adherence and virologic response were considered secondary outcomes.
Data extractionThe following data from included studies were collected: data on study design, baseline characteristics, and primary and secondary outcomes. Data included definition of adherence, methods of adherence assessment and adherence outcomes (mean adherence, predictors of poor adherence, correlation between adherence and virologic response).
DefinitionsMedication adherence depended on the definition in the study; however, adherence defined as dose reduction by physicians or early treatment discontinuation were not considered valid definitions (see inclusion and selection of studies).
Hepatitis B medication regimens for this review were defined as the nucleos(t)ide analogues (NUCs) approved for treatment, including LAM, ADV, ETV, TDF and TBV.8 Studies assessing adherence to PEG-IFN in HBV patients were not included since data were scarce and a uniform measure of treatment success is not available. Medication regimens for hepatitis C were defined as combination therapy, including PEG-IFN α2a or -α2b combined with ribavirin (RBV) or interferon (IFN) combined with RBV.5
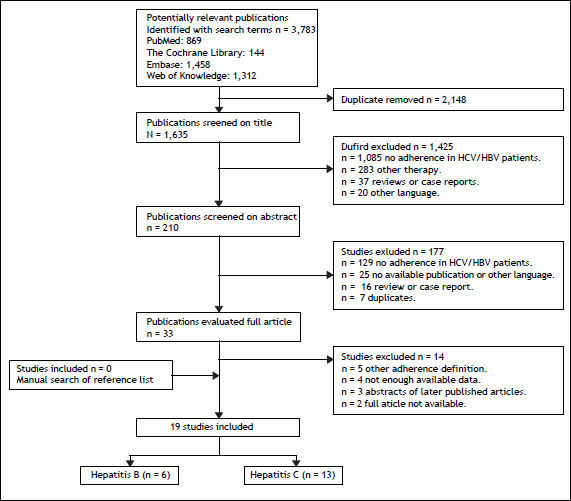
ResultsLiterature searchThe literature search yielded 3,783 publications, of which thirty-three relevant studies were evaluated as full article (Figure 1). After applying the inclusion criteria, a total of 19 articles and abstracts describing 17 original studies were included. Thirteen studies assessed adherence to HCV medication and six studies adherence to HBV medication.
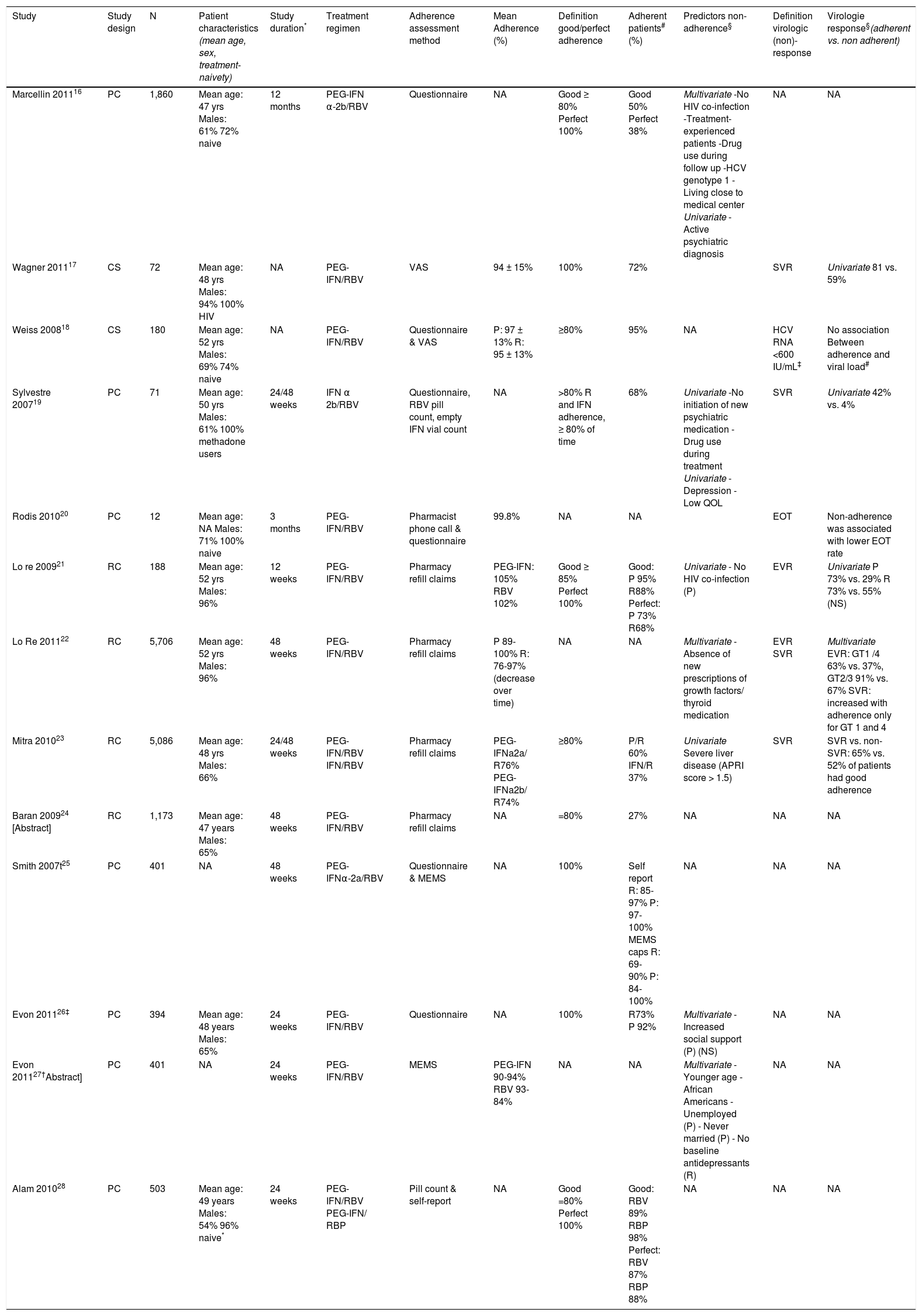
Hepatitis CThirteen studies assessed adherence to combination therapy in chronic hepatitis C patients16–28 (Table 1).
- •
Patient self assessment. Five studies measured adherence to a PEG-IFN/RBV or IFN/RBV regimen by means of patient self-reports.16–20 Marcellin16 reported that 50% of 1,860 French HCV patients exhibited good adherence (= 80%), whereas 38% exhibited perfect adherence (100%). Factors associated with perfect adherence in multivariate analysis were: HIV co-infection (OR 2.5, 95% CI 1.3-4.7, p = 0.003), no illicit drug use during follow-up (OR 2.4, 95% CI 1.3-4.3, p = 0.005), HCV genotype 3 (OR 1.6, 95% CI 1.2-2.0, p = 0.016), treatment-naivety (OR 1.3, 95% CI 1.0-1.7, p = 0.028) and longer transport time to the medical center (OR 1.0, 95% CI 1-1.006, p = 0.024). Wagner17 reported a mean adherence to PEG-IFN/RBV of 94 ± 15% (range 10-100%) in 72 HCV-HIV co-infected patients, 72% of patients exhibited perfect adherence (100%). In univariate analysis perfect adherence was associated with absence of an active psychiatric diagnosis at baseline (38% vs. 67%, p < 0.05) and SVR achievement (81 vs. 59%, p < 0.05). In a cross-sectional survey among 180 patients, Weiss18 reported good adherence (= 80%) to PEGIFN/RBV in 95% of patients, with a mean adherence of 97 ± 13% for PEG-IFN and 95 ± 13% for RBV. Non-adherence to PEG-IFN was significantly related to non-adherence to RBV (OR: 163, 95% CI 23-1,164, p < 0.001). No significant association between adherence and the latest viral load (also self-reported), achievement of undetectable viral load, demographic or treatment-related factors was found. Sylvestre19 reported that 68% of the 71 methadone-maintained patients in their study exhibited good adherence (= 80%) to IFN/RBV. In univariate analysis, good adherence was associated with initiation of new psychiatric medication in patients without a preexisting psychiatric diagnosis (94 vs. 46%, p = 0.04) and with no use of heroin, cocaine and/ or amphetamine during treatment (25 vs. 74%, p = 0.03). Among patients with good adherence SVR rates were higher (42 vs. 4%, p = 0.001). Rodis20 reported a mean adherence of 99.8% in 12 patients who were referred to an interdisciplinary HCV education and monitoring service. Non-adherence was associated with depression (p = 0.02), low quality of life (p = 0.045) and less chance of achieving end of treatment response (p = 0.048) in univariate analysis.
- •
Pharmacy claims. Four studies used pharmacy claims to measure adherence to HCV combination therapy and calculated adherence using the medication possession ratio (MPR).21–24 MPR generally is defined as the sum of day’s supply of a particular drug in an observed period divided by the number of days in this period. Lo Re21,22 measured adherence to PEG-IFN/RBV in two cohorts of HCV patients in the United States. In the first cohort of 188 patients,21 adherence was not truncated at 100% to evaluate trends in virologic outcomes at the highest observed levels of ad-herence. Good adherence (= 85%) to PEGIFN and RBV was observed in 95 and 88% of patients, respectively, with a mean adherence of 105% for PEG-IFN and 102% for RBV. Patients with HIV co-infection exhibited higher median adherence rates to PEG-IFN (110 vs. 103%, p = 0.01) and RBV (110 vs. 103%, p = 0.30). Adherence was not associated with alcohol abuse, posttraumatic stress disorder and depression. Patients with good adherence had a 0.7 log higher decrease in viral load at 12 weeks (3.2 vs. 2.6 log IU/mL, p = 0.04) and higher EVR rates (PEG-IFN: 73 vs. 29%, p = 0.02; RBV: 73 vs. 55%, p = 0.08). In a second cohort of 5086 patients Lo Re22 reported a mean PEG-IFN adherence of 100, 95, 95 and 89% for treatment weeks 0-12, 12-24, 24-36 and 36-48, respectively. The mean RBV adherence for the same periods was 97, 86, 84 and 76%, respectively. A mean adherence decrease of 7% for RBV and 3% for PEG-IFN per 12-week interval was observed (p > 0.001). Multivariate analysis showed an association between EVR rates and PEG-IFN/RBV adherence rates (p < 0.001, corrected for age, race and site). Higher adherence was associated with SVR achievement in patients with genotype 1 or 4, but not for genotype 2 or 3. Mitra23 reported a mean adherence of 76% for PEG-IFNa2a/RBV and 74% for PEG-IFNa2b/RBV in 5,086 patients, 60% of the study population exhibited good adherence (= 80%). Patients with good adherence were more likely to have mild or moderate disease (65/62 vs. 50%) and to have higher total HCVrelated costs ($20.132 vs. $12.259, p < 0.01) but lower costs when pharmacy costs were excluded ($1,370 vs. $2,463, p < 0.01). Among patients who achieved SVR, significantly more patients exhibited good ad-herence (65 vs. 52%) compared to non-SVR patients. Baran24 quantified the impact of adherence to PEG-IFN/RBV on HCV-rela-ted complications after treatment in 1,173 patients. Good adherence (= 80%) was observed in 27% of the patients and was significantly associated with a lower probability of cancer (OR 1.4, 95% CI 1.3-1.7), anemia (OR 1.6, 95% CI 1.3-1.9), depression (OR 1.5, 95% CI 1.2-1.8) and use of resources (1.6, 95% CI 1.3-1.8) at 4 years follow-up. Three studies used data of (a subgroup of) a prospective cohort study primarily aimed at comparing SVR rates between 401 Caucasian and African American HCV patients treated with PEG-IFN alffa-2a/RBV.25–27 Adherence was measured by a questionnaire and electronic monitors inside prescription bottles (MEMS caps). Smith25 assessed the validity of these two adherence assessment methods. The percentage of patients with self-reported perfect adherence (100%) decreased over time and was 97-85% for RBV and 100-97% for PEG-IFN. The proportion of patients with partial or perfect adherence as measured by MEMS caps also decreased over time and was 90-69% for RBV and 100-84% for PEG-IFN. The two adherence methods agreed in 52-81% of the RBV cases (p < 0.05) and corresponded in general for more than 93% of cases for PEG-IFN. In the cases with disagreement, patients reported higher adherence rates in self-report than was reported by MEMS. Evon26 evaluated the effect of social support on adherence (based on the questionnaire) in 394 of 401 patients. Mean adherence to RBV and PEG-IFN was 73% and 92%, respectively. Adherence to PEG-IFN decreased as social support increased (RR = 0.98; 99% CI: 0.95, 1.001, p = 0.012), but adherence to RBV was not associated with social support. In a second study, Evon27 identified patient characteristics associated with treatment non-adherence. Based on MEMS caps data, mean adherence to PEG-IFN and RBV was 90–94% and 93-84%, respectively. Risk factors for non-adherence were different for PEG-IFN and RBV (Table 1).
In a prospective, observational study Alam28 compared adherence to RBV with adherence to RibaPak® (RBV tablets available in 400 mg and 600 mg), which could be associated with improved adherence compared with traditional ribavirin given the reduced pill burden. The proportion of patients with good adherence (= 80%) was higher among RibaPak® users compared to those using RBV (98 vs. 89%, p = 0.005). Mean missed doses (1.1 vs. 0.4, p = 0.01) and mean missed medication milligrams (47 mg vs. 15 mg, p = 0.01) during the 4 weeks prior to the week-24 visit were significantly higher for RBV compared to RibaPak®. Adherence to therapy was reported to be higher in patient self-reports compared to the pill count results.
- •
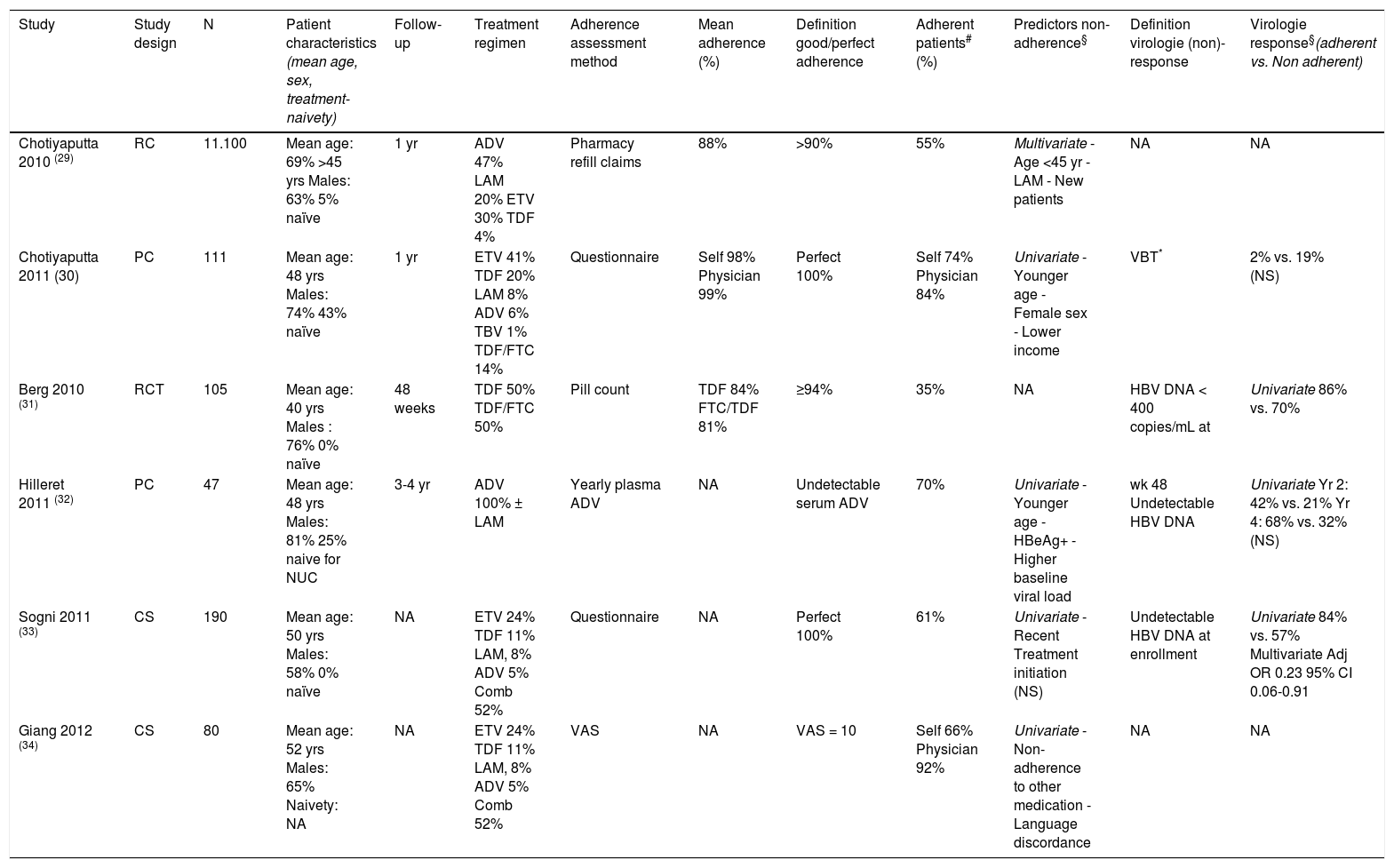
Hepatitis B. We identified six studies that assessed adherence to NUC regimens in HBV patients 29–34 (Table 2). Chotiyaputta29,30 assessed adherence in two cohorts of HBV patients in the United States. Using a national pharmacy refill claims database29 adherence of 11.100 HBV patients on various NUCs was assessed; a mean adherence of 88 ± 19% was reported. Independent predictors of good adherence (> 90%) were NUC treatment prior to study enrollment (57 vs. 50%, p = 0.03), age above 45 years (60 vs. 55%, p = 0.002) and NUCs other than LAM (60% vs. 51%, p < 0.001). In a second study Chotiyaputta30 prospectively assessed adherence by self-report in 111 patients at a single center. Mean adherence at enrolment was 98 ± 4%, which remained stable during the year of follow-up. Perfect adherence was reported in 74% of patients and was associated with male sex (82 vs. 53%, p = 0.006), older age (49 vs. 43 years, p = 0.02), and higher income (30% in $20-60,000 vs. 44% in > $100,000, p = 0.04). Perfect adherence was not associated with race, duration of HBV infection, type of HBV medication, history of prior HBV treatment, co-morbidities, number of oral medications, education and occupation. Undetectable HBV DNA levels were observed in 71% of patients with perfect adherence, compared to 77% of patients with < 100% ad-herence (p = 0.78). However, in the patients who completed 3 questionnaires, viral breakthrough was observed in 2% of patients with perfect adherence on all three questionnaires, in 6% with < 100% ad-herence on one questionnaire and in 19% with < 100% adherence on 2 or 3 questionnaires (p = 0.06). Patients reported higher adherence rates than physicians (perfect adherence 89 vs. 98%). In a randomized controlled trial in 105 patients who had an incomplete response to ADV, Berg31 compared efficacy and adherence to TDF and FTC/TDF therapy during 48 weeks. Median adherence rates, as assessed by pill counts, were similar in both groups (84% for TDF vs. 81% for TDF/FTC). Patients with high adherence (= 94%) were more likely to have HBV DNA < 400 copies/mL at week 48 compared to those with low adherence (= 68%). In 47 patients using ADV with or without LAM, Hilleret32 evaluated adherence by measurement of ADV plasma levels (yearly and when HBV DNA level increased with > 0.5 log IU/mL). Thirty percent of the patients were at least once non-adherent to ADV treatment (undetectable ADV level). Patients with good adherence were older (51 years vs. 39 years, p < 0.001), more frequently HBeAg negative (82 vs. 43%) and had lower baseline viral load (6.1 vs. 7.4 log IU/mL). Good adherence was associated with a lower mean viral load after 1 year of treatment (3.0 vs. 4.4 log IU/mL; p = 0.001), and higher rates of undetectable HBV DNA levels (42 vs. 21% after 2 years (p < 0.01); 68% vs. 32% after 4 years (p < 0.07)). Sogni33 performed a cross-sectional survey in 190 HBV patients using a single questionnaire with VAS. The proportions of patients with perfect ad-herence, moderate adherence (1 skipped dose or VAS 8.1-9.9) and non-adherence were 61, 32 and 7%, respectively. In patients with perfect adherence, previous treatment duration was longer (p = 0.085) and HBe-loss occurred more frequently in HBe-positive patients (46 vs. 25-38%, p = 0.486) compared to less adherent patients. Adherence was not related to geographical origin, baseline viral load, first/second line treatment and mono/combination therapy. In multivariate analysis, perfect adherence was an independent predictor of complete virological suppression at enrollment. Giang34 determined adherence to various NUCs amongst 80 HBV patients and their physicians using a VAS. Sixty-six percent of the patients reported optimal adherence (VAS = 10), while 92% of physicians thought their patients had optimal adherence. Suboptimal adherence (VAS = 9) was significantly associated with suboptimal adherence to other medicaments (p = 0.04) and language-discordance between physician and patient (p = 0.04). No significant associations between adherence level and sex, age, country of birth and ethnicity were observed.
Table 2.Overview of adherence studies in hepatitis B.
Study Study design N Patient characteristics (mean age, sex, treatment-naivety) Follow-up Treatment regimen Adherence assessment method Mean adherence (%) Definition good/perfect adherence Adherent patients# (%) Predictors non-adherence§ Definition virologie (non)-response Virologie response§(adherent vs. Non adherent) Chotiyaputta 2010 (29) RC 11.100 Mean age: 69% >45 yrs Males: 63% 5% naïve 1 yr ADV 47% LAM 20% ETV 30% TDF 4% Pharmacy refill claims 88% >90% 55% Multivariate - Age <45 yr - LAM - New patients NA NA Chotiyaputta 2011 (30) PC 111 Mean age: 48 yrs Males: 74% 43% naïve 1 yr ETV 41% TDF 20% LAM 8% ADV 6% TBV 1% TDF/FTC 14% Questionnaire Self 98% Physician 99% Perfect 100% Self 74% Physician 84% Univariate - Younger age - Female sex - Lower income VBT* 2% vs. 19% (NS) Berg 2010 (31) RCT 105 Mean age: 40 yrs Males : 76% 0% naïve 48 weeks TDF 50% TDF/FTC 50% Pill count TDF 84% FTC/TDF 81% ≥94% 35% NA HBV DNA < 400 copies/mL at Univariate 86% vs. 70% Hilleret 2011 (32) PC 47 Mean age: 48 yrs Males: 81% 25% naive for NUC 3-4 yr ADV 100% ± LAM Yearly plasma ADV NA Undetectable serum ADV 70% Univariate - Younger age - HBeAg+ - Higher baseline viral load wk 48 Undetectable HBV DNA Univariate Yr 2: 42% vs. 21% Yr 4: 68% vs. 32% (NS) Sogni 2011 (33) CS 190 Mean age: 50 yrs Males: 58% 0% naïve NA ETV 24% TDF 11% LAM, 8% ADV 5% Comb 52% Questionnaire NA Perfect 100% 61% Univariate - Recent Treatment initiation (NS) Undetectable HBV DNA at enrollment Univariate 84% vs. 57% Multivariate Adj OR 0.23 95% CI 0.06-0.91 Giang 2012 (34) CS 80 Mean age: 52 yrs Males: 65% Naivety: NA NA ETV 24% TDF 11% LAM, 8% ADV 5% Comb 52% VAS NA VAS = 10 Self 66% Physician 92% Univariate - Non-adherence to other medication - Language discordance NA NA *VBT was defined as an increase in HBV DNA by ≥ 1 log10 above nadir or 10 times the lower limit of detection in patients who had undetectable HBV DNA previously ADV: adefovir. CS: cross-sectional. ETV: entecavir. HBeAg+: hepatitis B e antigen positivity. LAM: lamivudine. NA: not applicable. PC: prospective cohort. RC: retrospective cohort. RCT: randomized controlled trial. TDF: tenofovir. TDF/FTC: tenofovir/emtricitabine. VAS: visual analogue scale. VBT: virological breakthrough, yrs: years,
Overview of studies assessing adherence in hepatitis C patients.
| Study | Study design | N | Patient characteristics (mean age, sex, treatment-naivety) | Study duration* | Treatment regimen | Adherence assessment method | Mean Adherence (%) | Definition good/perfect adherence | Adherent patients# (%) | Predictors non-adherence§ | Definition virologic (non)-response | Virologie response§(adherent vs. non adherent) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marcellin 201116 | PC | 1,860 | Mean age: 47 yrs Males: 61% 72% naive | 12 months | PEG-IFN α-2b/RBV | Questionnaire | NA | Good ≥ 80% Perfect 100% | Good 50% Perfect 38% | Multivariate -No HIV co-infection -Treatment-experienced patients -Drug use during follow up -HCV genotype 1 -Living close to medical center Univariate - Active psychiatric diagnosis | NA | NA |
| Wagner 201117 | CS | 72 | Mean age: 48 yrs Males: 94% 100% HIV | NA | PEG-IFN/RBV | VAS | 94 ± 15% | 100% | 72% | SVR | Univariate 81 vs. 59% | |
| Weiss 200818 | CS | 180 | Mean age: 52 yrs Males: 69% 74% naive | NA | PEG-IFN/RBV | Questionnaire & VAS | P: 97 ± 13% R: 95 ± 13% | ≥80% | 95% | NA | HCV RNA <600 IU/mL‡ | No association Between adherence and viral load# |
| Sylvestre 200719 | PC | 71 | Mean age: 50 yrs Males: 61% 100% methadone users | 24/48 weeks | IFN α 2b/RBV | Questionnaire, RBV pill count, empty IFN vial count | NA | >80% R and IFN adherence, ≥ 80% of time | 68% | Univariate -No initiation of new psychiatric medication -Drug use during treatment Univariate - Depression - Low QOL | SVR | Univariate 42% vs. 4% |
| Rodis 201020 | PC | 12 | Mean age: NA Males: 71% 100% naive | 3 months | PEG-IFN/RBV | Pharmacist phone call & questionnaire | 99.8% | NA | NA | EOT | Non-adherence was associated with lower EOT rate | |
| Lo re 200921 | RC | 188 | Mean age: 52 yrs Males: 96% | 12 weeks | PEG-IFN/RBV | Pharmacy refill claims | PEG-IFN: 105% RBV 102% | Good ≥ 85% Perfect 100% | Good: P 95% R88% Perfect: P 73% R68% | Univariate - No HIV co-infection (P) | EVR | Univariate P 73% vs. 29% R 73% vs. 55% (NS) |
| Lo Re 201122 | RC | 5,706 | Mean age: 52 yrs Males: 96% | 48 weeks | PEG-IFN/RBV | Pharmacy refill claims | P 89-100% R: 76-97% (decrease over time) | NA | NA | Multivariate - Absence of new prescriptions of growth factors/ thyroid medication | EVR SVR | Multivariate EVR: GT1 /4 63% vs. 37%, GT2/3 91% vs. 67% SVR: increased with adherence only for GT 1 and 4 |
| Mitra 201023 | RC | 5,086 | Mean age: 48 yrs Males: 66% | 24/48 weeks | PEG-IFN/RBV IFN/RBV | Pharmacy refill claims | PEG-IFNa2a/ R76% PEG-IFNa2b/ R74% | ≥80% | P/R 60% IFN/R 37% | Univariate Severe liver disease (APRI score > 1.5) | SVR | SVR vs. non-SVR: 65% vs. 52% of patients had good adherence |
| Baran 200924 [Abstract] | RC | 1,173 | Mean age: 47 years Males: 65% | 48 weeks | PEG-IFN/RBV | Pharmacy refill claims | NA | =80% | 27% | NA | NA | NA |
| Smith 2007t25 | PC | 401 | NA | 48 weeks | PEG-IFNα-2a/RBV | Questionnaire & MEMS | NA | 100% | Self report R: 85-97% P: 97-100% MEMS caps R: 69-90% P: 84-100% | NA | NA | NA |
| Evon 201126‡ | PC | 394 | Mean age: 48 years Males: 65% | 24 weeks | PEG-IFN/RBV | Questionnaire | NA | 100% | R73% P 92% | Multivariate - Increased social support (P) (NS) | NA | NA |
| Evon 201127†Abstract] | PC | 401 | NA | 24 weeks | PEG-IFN/RBV | MEMS | PEG-IFN 90-94% RBV 93-84% | NA | NA | Multivariate - Younger age - African Americans - Unemployed (P) - Never married (P) - No baseline antidepressants (R) | NA | NA |
| Alam 201028 | PC | 503 | Mean age: 49 years Males: 54% 96% naive* | 24 weeks | PEG-IFN/RBV PEG-IFN/ RBP | Pill count & self-report | NA | Good =80% Perfect 100% | Good: RBV 89% RBP 98% Perfect: RBV 87% RBP 88% | NA | NA | NA |
baseline and most recent viral load were gathered by patient self-reports (49% of patients reported this).
these 3 studies used data from the same prospective cohort study. APRI: AST platelet ratio index. CS: cross sectional. EOR: end of treatment response. EVR: early virologic response. GT: genotype. IFN: interferon. NA: not applicable. NS: not significant. MEMS: Medication Event Management System. mo: months. PC: prospective cohort. P/PEG-IFN: pegylated interferon. QOL: quality of life. R/RBV: ribavirin. RBP: Ribapak (RBV in 400 mg and 600 mg tablets). RC: retrospective cohort. SVR: sustained virologic response. VAS: visual analogue scale. yrs: years.
For various chronic diseases, it has been shown that non-adherence to treatment is associated with increased morbidity, mortality and excessive costs. This systematic review evaluated the available literature on adherence to combination treatment (PEG-interferon plus ribavirin) for HCV and NUC regimens for HBV.
A total of 19 studies that examined adherence in viral hepatitis B or C were included in this systematic review; 13 of these focused on HCV combination treatment whereas 6 studies assessed adherence to NUC treatment in chronic HBV patients. In contrast to the studies in HCV patients, all studies in HBV patients were recently published (2010-now). Direct comparison of the various studies included in this systematic review was found to be difficult due to differences in patient populations, study design, method of adherence assessment and definitions used for good adherence and virological response. Nonetheless, some trends that underscore important aspects of adherence to treatment for chronic viral hepatitis could be observed.
For chronic HCV, the mean adherence reported in the various studies ranged from 74 to 100%.17,18,20–23,27 The highest adherence rates were reported in studies that used patient self-report to measure adherence,16–20,25,26,28 whereas the lowest rates were reported in studies using pharmacy refill claims.21–24 Most studies also reported the proportion of patients with good adherence (defined as ≥ 80%), which varied from 27 to 96% in the various studies.16,18,19,23,24 Six studies reported the proportion of patients with perfect adherence (defined as 100%) which varied from 38 to 100%.16,17,21,25,26,28 Only a minority of studies gave a rationale for their cut-off adherence level. In one study, a cut-off of 85% was chosen because virological response was constant above this level.21 In other studies a 80% cut-off was chosen in analogy with previous studies,16,18,19,23,24 i.e. the 80/80/80 rule, which was originally based on dose reductions by physicians due to side effects rather than missed doses by the patient.13 Furthermore, a cut-off level of 80% adherence is frequently used in adherence studies in other chronic diseases.35–37
A majority of HCV studies also assessed possible predictors of non-adherence. Most frequently identified predictors were related to psychiatric diagnoses or illicit drug use.16,17,19 Two studies showed adherence rates were higher among patients with HIV co-infection.16,21 Demographic risk factors for non-adherence identified by a single study were younger age, African-American race, unemployment and being unmarried.27 Almost half of the HCV studies also investigated the association between adherence and virological response and all but one found a significant association.17–23 Only Weiss, et al. did not report a significant association.18 However, limitations of this study were the cross-sectional design, assessing patients at a large range of duration of antiviral treatment, and the collection of baseline and most recent HCV viral load by self-report. Adherence to treatment for chronic HCV will become an even more important issue with the recent introduction of protease inhibitors boceprevir and telaprevir for HCV genotype 1 infection.6,7 Treatment regimens for triple therapy are more complex and adherence to all three medications will therefore become more challenging for patients.
Compared to HCV studies, it is more difficult to draw conclusions about adherence in HBV patients since no common adherence definition was used in the six included studies. Mean adherence to the various NUC regimens was reported in three studies and varied from 81 to 99%.29–31 The proportion of patients with perfect adherence (100%) was also reported in three studies and varied from 66 to 92%.29,33,34 Overall, reported adherence rates were very high in all but one study. The highest adherence rates were observed in studies which used self-report or physician report. Interestingly, the lowest adherence rates were reported in the study by Berg, et al., a randomized controlled trial in which adherence was assessed by pill counts.31 Generally, adherence rates in randomized controlled trials are thought to be higher than in daily clinical practice due to frequent follow-up and highly motivated patients. Four HBV studies also assessed the possible association between adherence and virological response.30–33 All found virological response to be better in adherent patients compared to non-adherence patients, but not all differences were statistically significant. Predictors for non-adherence to NUC treatment for chronic HBV were younger age, recent treatment initiation, use of lamivudine (compared to other NUCs) and female sex.30,32–34
The main differences between the various studies included in this review were the methods used for adherence assessment and the number of patients, which are directly related. In total 5 different methods were used. Self-report, either by a questionnaire/VAS or directly to a health care worker was used in half of the studies. The use of self-reports to measure adherence is inexpensive and simple; however patients tend to overestimate their medication adherence.10 Indeed, in the studies included in our systematic review that used self-report, adherence rates were higher than in studies that used other methods of adherence assessment. Although there is no golden standard for measuring adherence and all adherence methods have limitations,10 the most accurate way of measurement is direct observation. None of the studies included in our systematic review used this method, most likely because direct observation is rather impractical and is almost exclusively used in randomized controlled trials. A more practical method in cohort studies is the use of electronic monitors which deliver precise and reliable adherence data. However, this method is rather expensive and therefore difficult to use in studies with large patient numbers. In our systematic review, only one original study (described in 2 separate articles) used electronic monitors placed in prescription bottles (MEMS caps).25,27 The use of pharmacy refill claims data is an easy and objective method to measure adherence in large patient groups. It was used in five studies included in our review with study populations ranging from 188 to 11,100 patients. Most important limitation of this method is the possibility of administrative errors and subsequent misclassification.10 Only one study measured drug levels in blood as a proxy for adhe-rence.32 It is one of the only direct measures of adherence. However, it is expensive and not available for all drugs. Furthermore, pharmacokinetics are also influenced by age, gender, renal function and variations in metabolism.
Our results suggest that adherence to treatment for chronic viral hepatitis appears to be somewhat better, compared to other chronic diseases. For example, mean adherence rates in HIV patients treated with highly active antiretroviral therapy are around 60%.38,39 In patients treated with protonpump inhibitors for gastroesophageal reflux disease, the proportion of patients with > 80% adherence ranged from 54 to 68%.36
Our systematic review has several strengths and some limitations. We are the first to summarize the available literature on adherence in both HBV and HCV. This information is relevant for clinicians treating patients with chronic viral hepatitis. However, it should be noted that we only included studies that evaluated adherence in HCV patients treated with PEG-IFN/RBV combination therapy or HBV patients treated with NUCs. Therefore, we do not have any data regarding other medication regimens in HCV and HBV patients, for example for PEG-IFN treatment for HBV and triple therapy with protease inhibitors for HCV patients. The studies included in our systematic review were heterogeneous, both in terms of study design and patient characteristics as well as definitions of adherence and virological response. Each of these factors could be a source of bias, it is therefore not possible to merge the available data and give an estimate of adherence during HCV and HBV treatment. Future research on this topic should therefore focus on the establishment and validation of adherence assessment methods that are accurate, not expensive and easy to use in clinical practice. Future studies should also further elucidate predictors of non-adherence and develop strategies to optimize adherence.
ConclusionThe available evidence suggests that non-adherence in chronic viral hepatitis could be a less frequent phenomenon than in other chronic diseases. However, given the increased risk of virological failure in poor adherent patients, clinicians should routinely address adherence issues in all patients treated for chronic viral hepatitis.
Abbreviations- •
ADV: adefovir.
- •
CHC: chronic hepatitis C.
- •
CHB: chronic hepatitis B.
- •
ETV: entecavir.
- •
EVR: early virologic response.
- •
HCV: hepatitis C virus.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HIV: human immunodeficiency virus.
- •
IFN: interferon.
- •
LAM: lamivudine.
- •
MEMS: Medication Event Management System.
- •
MPR: medication possession ratio.
- •
NUC: nucleos(t)ide analogue.
- •
PEG-IFN: pegylated interferon.
- •
RBV: ribavirin.
- •
SVR: sustained virologic response.
- •
TBV: telbivudine.
- •
TDF: tenofovir disoproxil.
- •
VAS: visual analogue scale.
Authors’ declaration of personal interests: Karel J. van Erpecum has served as a speaker, a consultant or an advisory board member for Bristol-Meyers Squibb BV, Gilead BV and Schering-Plough BV (MSD).The other authors declare that they have no competing financial interests.
Declaration of Funding InterestsNo specific funding has been provided for this study.