
Special issue on hepatocellular carcinoma (HCC) and hepatitis B and C as its main causes worldwide
Más datosImproving the prognosis of patients with hepatocellular carcinoma (HCC) undergoing hepatectomy is critical. This article aims to investigate the risk factors affecting the prognosis of HCC patients with Child-Pugh A (CPA) liver function after hepatectomy and to compare the prognosis of patients with anatomical resection (AR) and nonanatomical resection (NAR).
MethodsIn total, 186 patients diagnosed with HCC between 2013 and 2019 were retrospectively enrolled. Univariate and multivariate analyses were performed using a Cox proportional hazard regression model to explore the factors related to prognosis. Overall survival (OS) and progression-free survival (PFS) were analyzed by log-rank tests and are shown by Kaplan-Meier curves. Chi-square tests and Mann-Whitney U tests were used to compare the difference in clinical characteristics between AR and NAR patients.
ResultsAmong the 186 enrolled patients, only 73 were followed over 60 months. The 1-, 3-, and 5-year survival rates were 74.5%, 46.7% and 26.0%, respectively. Multivariate analyses demonstrated that portal vein invasion (PVI) and tumor size were independent risk factors for OS and PFS. Preoperative hepatitis B surface antigen (HBsAg) and a-fetoprotein (AFP) levels were identified as independent risk factors only for PFS. In univariate analysis, the NAR group had a better OS rate than the AR group (1-year: 80.4% vs. 63.6%, 3-year: 55.9% vs. 30.3%, 5-year: 34.8% vs. 11.1%), but this was not confirmed by multivariate analysis.
ConclusionsPVI and tumor size > 5 cm are risk factors for the prognosis of CPA HCC patients after hepatectomy, but the surgical type is not.
hepatocellular carcinoma;
Child-Pugh A
anatomical resection
nonanatomical resection
overall survival
progression-free survival
portal vein invasion
hepatitis B surface antigen
a-fetoprotein
indocyanine green retention rate at 15 minutes
computed tomography
transarterial chemoembolization
hepatitis C virus
anti-hepatitis B virus therapy
alanine transaminase
aspartate aminotransferase
total bilirubin
total protein
albumin
hazard ratio
95% confidence interval
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and ranks fourth in mortality [1]. Surgical resection is a widely accepted potential curative therapy for HCC, especially for CPA liver function, but a high recurrence rate is a major problem for these patients [2, 3]. Therefore, a large number of studies have been conducted to identify important predictors of prognosis after curative hepatectomy. To improve the outcome of surgical resection, AR, which completely removes the tumor-bearing areas, contributes to prolonging OS and reducing recurrence, especially for locally invasive masses [4]. However, some studies showed no difference between AR and NAR [5, 6]. The prognostic factors after hepatectomy are different depending on the medical units or institutions, and the influence of surgical type on prognosis is still controversial.
This study aimed to identify the risk factors related to OS and postoperative recurrence and to compare AR and NAR postoperation in resectable CPA HCC in our institution.
2Materials and Methods2.1PatientsA total of 186 patients with HCC who had undergone curative hepatectomy were confirmed pathologically between 2013 and 2019 at The Second Hospital of Anhui Medical University. Preoperative hepatic function was evaluated with Child-Pugh classification and indocyanine green retention rate at 15 minutes (ICG15) for patients with potentially curative resection. All tumor sizes were measured by preoperative CT/MRI, and further confirmed by measurement of specimen post-operation. The size of tumor was defined as the sum of diameter of each nodule. The preoperative diagnosis of portal invasion was made by enhanced CT/MRI imaging which was further confirmed by pathology which is defined as tumor cells invading the portal vein vessels according to the Liver Cancer Study Group of Japan (LCSGJ) [7]. The criteria we used for the curative purpose was China liver cancer staging criteria (CNLC) which is China's clinical guidelines for managing HCC [8]. AR was defined as complete removal of the tumor-involved anatomic section together with its portal vein branch, which included right or left hemihepatectomy and left lateral sectionectomy. Ultrasound-guided and hilar approach ligation of target Glissonean pedicles were used to visualize the cut boundary. NAR was defined as a surgical margin at least 1 cm from the tumor unless the mass was attached to the retained hepatic vein or Glissonean pedicle. The last day of follow-up was March 1, 2020. The median time of follow-up was 25 months. Postoperative follow-up data included AFP level and 1-, 3-, and 5-day postoperative serum hepatic function analysis. Ultrasonography combined with computed tomography (CT) or magnetic resonance imaging was performed every 3 months. Recurrence was confirmed by imaging findings and AFP levels. Recurrent HCC was treated by repeat hepatectomy, transarterial chemoembolization (TACE), and radiofrequency ablation, as appropriate. This study was approved by the ethics committee of The Second Hospital of Anhui Medical University.
2.2Inclusion and exclusion criteriaThe inclusion criteria were as follows: (a) No treatment for HCC was performed before the surgery; (b) Good preoperative hepatic function: Child-Pugh classification is A and ICG15<20%; (c) Diagnosis of HCC was confirmed by pathology post-operation; and (d) No evidence of extrahepatic metastasis. Exclusion criteria: (a) incomplete clinical and follow-up data, (b) palliative hepatic resection, (c) death from non-HCC-related disease or accident, and (d) history of other malignant tumors.
2.3Clinicopathological factorsThe following clinicopathological factors were selected for analysis: sex, age, alcohol consumption, HBsAg, hepatitis C virus (HCV), anti-hepatitis B virus therapy (anti-HBV), cirrhosis, AFP, alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), total protein (TP), albumin (ALB), operation time, blood loss, portal vein invasion (PVI), tumor number, tumor diameter, hospital stay, and complications. As portal hypertension is more common in HCC patients in east Asia countries than that in western countries, it is not considered as prerequisite for HCC management guidelines in China and Japan [8, 9]. Here, the status of portal hypertension is not evaluated.
2.4Statistical analysisThe data distribution was tested with Kolmogorov-Smirnov analysis. The clinicopathological factors were compared by the Mann-Whitney U test and chi-squared test. The OS and PFS in the two groups were analyzed with the Kaplan-Meier method using log-rank tests. Univariate and multivariate analyses were employed to evaluate the prognostic factors using a Cox proportional hazard model. Statistical differences were identified as a P value less than 0.05. SPSS software package version 21.0 was used for data analysis.
3Results3.1Clinicopathological characteristics and univariate and multivariate analysis of risk factorsClinicopathological characteristics are shown in Table 1. In univariate analysis, AFP level, surgical type, PVI status, and tumor size were statistically significant for OS (Table 2). AFP level, positive or negative HBsAg, PVI or not, and tumor size were statistically significant for PFS (Table 3). In multivariate analysis, PVI (p = 0.022) and tumor size (p < 0.001) were both identified as independent risk factors for OS. PVI (p = 0.049), tumor size beyond 5 cm preoperatively (p < 0.001), AFP > 400 ng/mL (p = 0.015) and positive HBsAg (p = 0.003) were effective in predicting HCC recurrence.
Clinicopathological characteristics of the included patients
Univariate and multivariate analyses of prognostic factors for OS after hepatectomy
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Factors | HR | 95% CI | p value | HR | 95% CI | p value |
| Sex (male/female) | 0.81 | 0.408∼1.610 | 0.548 | |||
| Age (≤65/>65 years) | 1.02 | 0.547∼1.896 | 0.953 | |||
| Drink (yes/no) | 1.42 | 0.820∼2.443 | 0.213 | |||
| AFP (≤400/>400 ng/ml) | 0.53 | 0.308∼0.897 | 0.018* | 0.62 | 0.359∼1.074 | 0.088 |
| Cirrhosis (yes/no) | 1.51 | 0.830∼2.735 | 0.178 | |||
| HBsAg (yes/no) | 1.87 | 0.842∼4.116 | 0.125 | |||
| Anti-HBV (yes/no) | 0.89 | 0.524∼1.521 | 0.677 | |||
| PVI (yes/no) | 3.18 | 1.770∼5.698 | < 0.001* | 2.05 | 1.106∼3.784 | 0.022* |
| Surgical type (AR/NAR) | 1.77 | 1.033∼3.019 | 0.038* | 1.17 | 0.665∼2.052 | 0.589 |
| Tumor number (S/M) | 1.30 | 0.515∼3.244 | 0.584 | |||
| Tumor diameter (≤5/>5 cm) | 0.25 | 0.143∼0.449 | < 0.001* | 0.31 | 0.168∼0.556 | < 0.001* |
HR hazard ratio, 95% CI 95% confidence interval
Univariate and multivariate analyses of prognostic factors for PFS after hepatectomy
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Factors | HR | 95% CI | p value | HR | 95% CI | p value |
| Sex (male/female) | 0.95 | 0.547∼1.653 | 0.858 | |||
| Age (≤65/>65 years) | 1.15 | 0.708∼1.872 | 0.569 | |||
| Drink (yes/no) | 1.14 | 0.744∼1.751 | 0.546 | |||
| AFP (≤400/>400 ng/ml) | 0.52 | 0.343∼0.786 | 0.002* | 0.59 | 0.388∼0.901 | 0.015* |
| Cirrhosis (yes/no) | 1.02 | 0.664∼1.571 | 0.923 | |||
| HBsAg (yes/no) | 2.23 | 1.187∼4.202 | 0.013* | 2.66 | 1.395∼5.083 | 0.003* |
| Anti-HBV (yes/no) | 1.41 | 0.936∼2.110 | 0.101 | |||
| PVI (yes/no) | 2.26 | 1.368∼3.718 | < 0.001* | 1.69 | 1.003∼2.832 | 0.049* |
| Surgical type (AR/NAR) | 1.17 | 0.765∼1.786 | 0.471 | |||
| Tumor number (S/M) | 0.87 | 0.473∼1.591 | 0.646 | |||
| Tumor diameter (≤5/>5 cm) | 0.45 | 0.301∼0.682 | < 0.001* | 0.43 | 0.282∼0.667 | < 0.001* |
HR hazard ratio, 95% CI 95% confidence interval
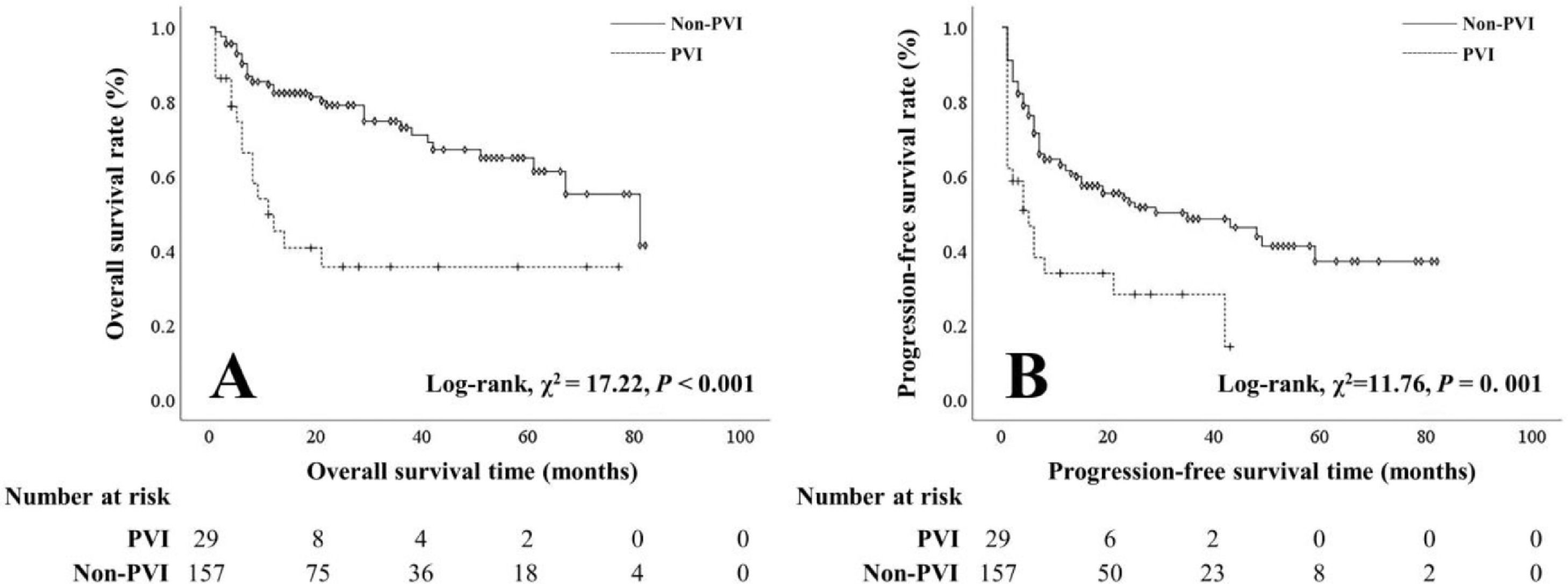
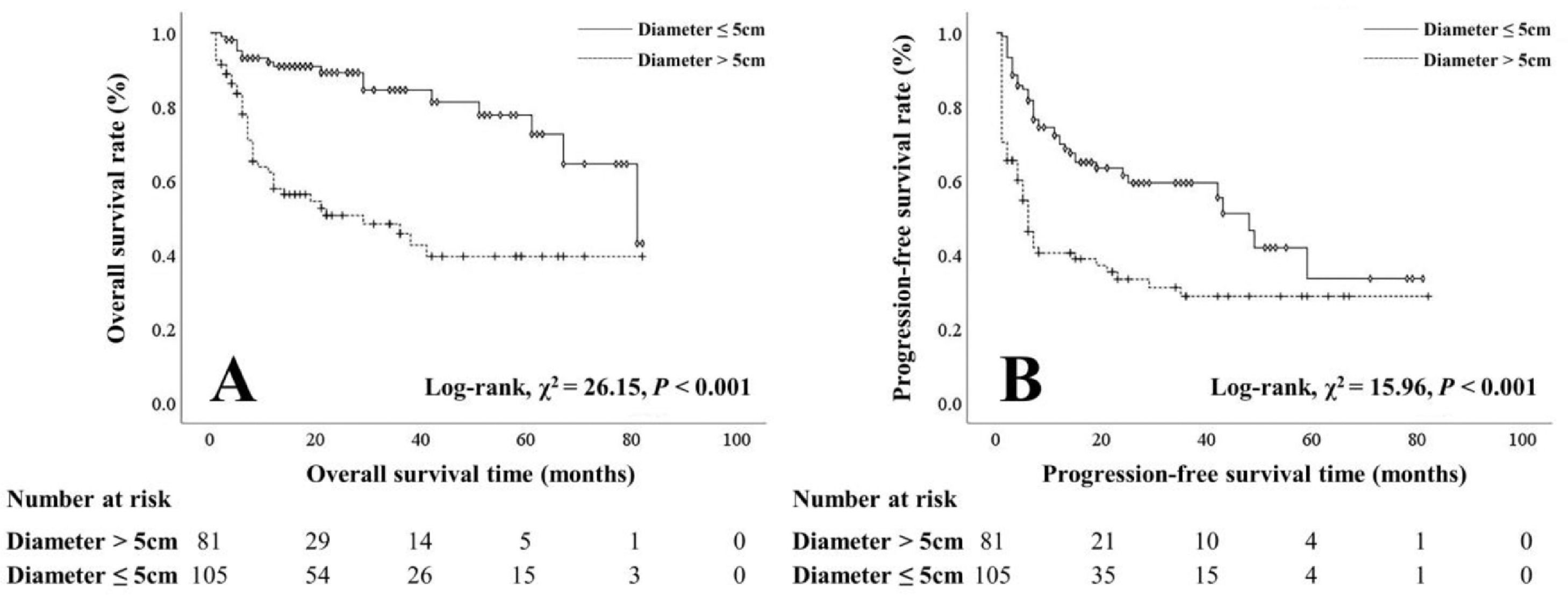
The OS and PFS rates of patients with PVI were worse than those of patients without PVI (1-year: 41.7% vs 80.5%, 3-year: 20.0% vs 54.2%, 5-year: 5.9% vs 30.9%; 1-year: 28.0% vs 57.7%, 3-year: 9.5% vs 26.3%, 5-year: 0.0% vs 8.6%) (Figure 1). Patients with smaller tumors (≤ 5 cm) had better OS and PFS rates than those with larger tumors (> 5 cm) (1-year: 89.8% vs 55.1%, 3-year: 70.0% vs 28.8%, 5-year: 48.3% vs 9.3%; 1-year: 66.3% vs 36.1%, 3-year: 31.5% vs 16.1%, 5-year: 6.7% vs 5.5%) (Figure 2).
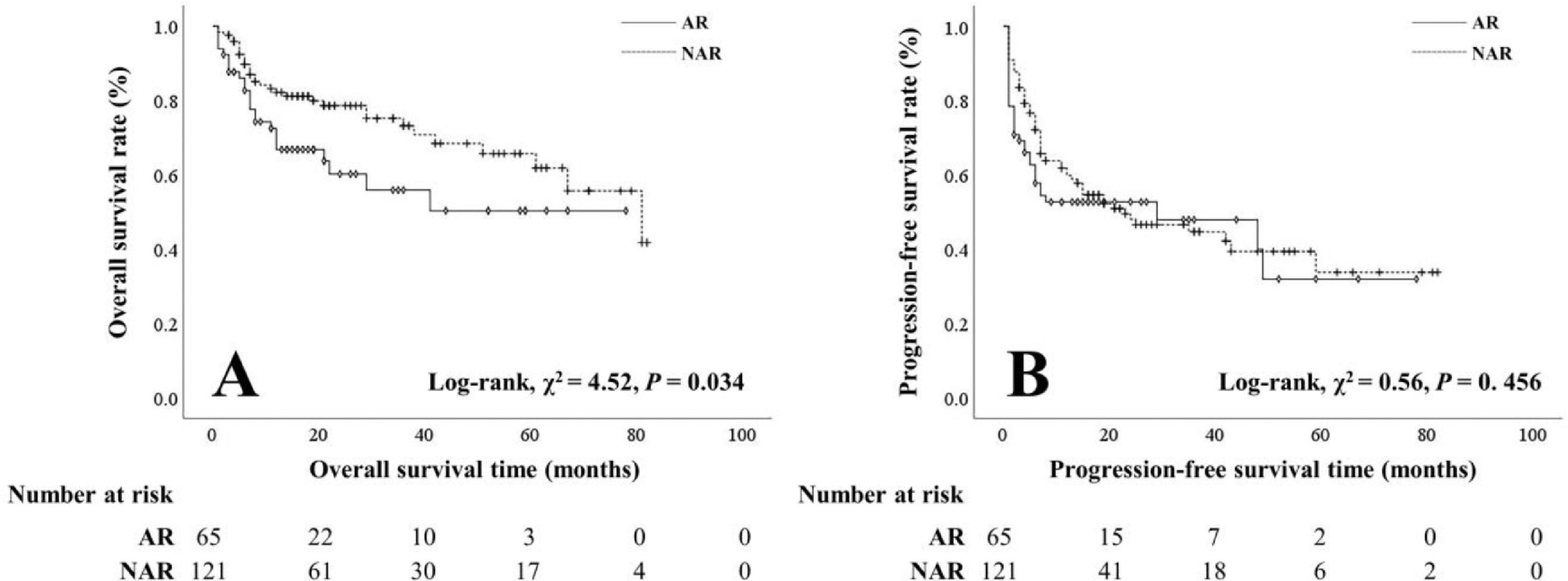
3.3Clinicopathological characteristics and prognosis of patients with either surgical approachNo significant difference was shown in age, sex, viral hepatitis, cirrhosis, preoperative hepatic function, serum AFP level, tumor number, intraoperative blood loss, postoperative hepatic function, or morbidity between the AR and NAR groups. More patients with PVI (24.6% vs 10.7%) and larger tumors (> 5 cm, 58.5% vs 35.5%) were observed in the AR group. Compared with the NAR group, the hospital stay was longer in the AR group (12.89 ± 6.92 VS 10.31 ± 5.15 months) (Table 4). Notably, the OS rate of the NAR group was better than that of the AR group (1-year: 80.4% vs 63.6%, 3-year: 55.9% vs 30.3%, 5-year: 34.8% vs 11.1%). However, AR was not an independent protective factor for prognosis. No significant difference was observed in the PFS rate between the NAR and AR groups (Figure 3).
Clinicopathological characteristics of patients with AR and NAR
| Surgical type | |||
|---|---|---|---|
| Factors | AR(n=65) | NAR(n=121) | p value |
| Sex (male/female) | 53/12 | 103/18 | 0.526 |
| Age (≤65/>65 years) | 49/16 | 91/30 | 0.979 |
| Drink (yes/no) | 22/43 | 40/81 | 0.913 |
| HBsAg (yes/no) | 50/15 | 101/20 | 0.276 |
| HCV (yes/no) | 3/62 | 5/116 | 1.000 |
| Anti-HBV (yes/no) | 29/35 | 57/62 | 0.738 |
| Cirrhosis (yes/no) | 45/20 | 80/41 | 0.666 |
| AFP (≤400/>400 ng/ml) | 36/29 | 84/37 | 0.056 |
| ALT (U/L) | 42.37 ±37.09 | 44.72 ± 47.80 | 0.937 |
| AST (U/L) | 48.18 ± 36.60 | 47.55 ± 50.64 | 0.110 |
| TB (μmol/L) | 20.52 ± 28.94 | 19.66 ± 20.39 | 0.420 |
| TP (g/L) | 66.33 ± 5.74 | 66.65 ± 6.75 | 0.687 |
| ALB (g/L) | 37.10 ± 5.89 | 37.79 ± 6.25 | 0.524 |
| Operation time (min) | 235.54 ± 118.38 | 202.45 ± 77.38 | 0.101 |
| Blood loss (ml) | 278.00 ± 245.48 | 236.24 ± 214.35 | 0.171 |
| PVI (yes/no) | 16/49 | 13/108 | 0.013* |
| Tumor number (s/m) | 60/5 | 104/17 | 0.201 |
| Tumor diameter (≤5/>5 cm) | 27/38 | 78/43 | 0.003* |
| Hospital stay (days) | 12.89 ± 6.92 | 10.31 ± 5.15 | 0.003* |
| Complications (yes/no) | 45/20 | 73/48 | 0.229 |
In recent decades, the potential clinical importance of anatomical resection in treating HCC has been advocated based on theoretical recognition of HCC biological behavior. AR, which completely removes tumor-bearing blood vessels, seems to be superior to NAR [10]. Many clinicopathological factors determine the oncogenesis and pathological progression of HCC. Whether advancement in local therapy skills and techniques in HCC could be translated into an oncological benefit is still debated [11].
In this retrospective study, univariate and multivariate Cox regression analyses showed that tumor diameter and PVI were independent risk factors for both OS and PFS. The surgical type was not an independent risk factor for prognosis. Interestingly, the NAR group had a better OS than the AR group based on univariate analysis. In the comparison of basic clinicopathological characteristics, we found a larger tumor size and a higher PVI incidence rate in the AR group than in the NAR group, suggesting that surgical technique improvement does not translate into oncological and survival benefits in relatively advanced-stage HCC. Tumor size is a widely recognized prognostic factor in HCC staging criteria, including the 8th American Joint Committee on Cancer, Barcelona Clinic Liver Cancer classification, and China liver cancer staging. Some studies claim that AR reduces patient recurrence and favors OS with tumors smaller than 5 cm [12, 13]. However, it is commonly reported that AR is not superior to NAR regarding survival benefit. Notably, in this study, patients with large tumors were more adapted to the AR surgical type because large tumors would need a wide cutting margin, which may cause survival bias. Tumors with macrovascular invasion are commonly regarded as a main pathological characteristic for poor prognosis. For patients with macroscopic PVI, AR is preferable because it is thought to maintain good liver function reserve and achieve R0 resection [6].
Two other commonly recognized factors were identified as risk factors for recurrence in this study: AFP and HBsAg. AFP > 400 ng/mL is a strong indication for recurrence and is associated with regulating proliferation, apoptosis, and autophagy and inhibiting the immune response of cells in HCC [14]. Chronic HBV hepatitis accounts for most cases of HCC in China. HBsAg is recognized as a risk factor for HCC recurrence. Hepatitis B surface antigen is derived mainly from the integrated form of HBV DNA [15]. HBV infections can be identified using serum HBV DNA, HBeAg, and HBV covalently closed circular DNA levels, which reflects not only active virus chronic inflammation in the liver but also initiates hepatocarcinogenesis through accumulating mutations caused by recurrent inflammation [16–18]. Successful clearance of HBsAg expression in HBV patients can increase survival time and reduce HCC recurrence [18]. In this study, we found that HBsAg was an independent risk factor for HCC recurrence postoperation. Effective clearance of HBsAg might provide increased survival benefit in these patients.
The limitations of this study include the following: (a) its retrospective design; (b) the enrolled patients did not all have full follow-up records; and (c) no propensity score matching was performed in the AR group and NAR group, which might induce selection bias.
In conclusion, the surgical type is not the dominant factor in the prognosis of CPA HCC patients undergoing hepatectomy. PVI and large tumor size (> 5 cm) indicate poor prognosis in patients.
Authors’ contributionsStudy concept and design: Xiao Cui, Dachen Zhou, Xiaoping Geng; Data acquisition: Sheng Wei, Minghao Yang, Hui Hou; Data analysis and interpretation: Xiao Cui, Minghao Yang; Drafted the manuscript: Sheng Wei, Minghao Yang; Revised the manuscript: Dachen Zhou, Xiao Cui; Final approval of the version to be submitted: Xiaoping Geng, Qiru Xiong
FundingThis work was supported by the Natural Science Foundations of both Anhui Province (1808085MH270) and the Higher Education Institutions of Anhui Province (KJ2017A825).
The publication of this article was funded by ROCHE. The funder did not intervene in or in any way influence the content of the text.





 PFS (B) in
PFS (B) in  PFS (B) in
PFS (B) in  PFS (B) in
PFS (B) in 



