Introduction. Type 1 hepatorenal syndrome (HRS) is a functional renal failure that complicates end-stage cirrhosis. The vasopressin analogue terlipressin has been associated with improved renal function in patients with type 1 HRS.
Aim. To evaluate the effectiveness of an infusion of terlipressin plus albumin in reversing type 1 HRS, its tolerability, and its adverse effects.
Methods. Thirteen consecutive patients with cirrhosis and type 1 HRS were included in the study. All patients received terlipressin plus albumin as treatment for HRS. The patients were divided in two groups. Group 1 contained eight patients in whom HRS was reversed with treatment, who were classified as responders. Group 2 contained five patients who were nonresponders.
Results.Sixty-one percent of the patients who received terlipressin plus albumin responded to therapy and underwent HRS reversal. In two patients, treatment with terlipressin was stopped because of adverse events. No relapse of HRS after terlipressin withdrawal was observed in this study.
Conclusion.The rate of successful treatment with terlipressin plus albumin was 61%, similar to that in previously reported controlled trials. However, this is the first experience reported in Mexico. A cardiovascular evaluation is required before the start of treatment with terlipressin. This treatment appears to be an effective therapy for improving renal function in patients with type 1 HRS.
Hepatorenal syndrome (HRS) is an entity observed in patients with chronic liver disease, advanced liver failure, and portal hypertension.1 It is characterized by impaired renal function and marked abnormalities in the arterial circulation, with activation of the endogenous vasoactive systems.2 The main pathophysiological change is intrarenal vasoconstriction, which reduces the renal flow and glomerular filtration rate in response to widespread arterial vasodilation, in the absence of pathological changes in the kidney tissue and preserved renal tubular function.3 In cirrhotic patients with ascites, prerenal failure (42%) and acute tubular necrosis (38%) represent the most common forms of acute renal failure, whereas HRS occurs in 20% of cirrhotic patients. The incidence of HRS in patients with cirrhosis and ascites after the first year is 18%, reaching 39% after five years.4
The International Ascites Club (IAC) has classified HRS into two types.5 Type 1 HRS is characterized by severe and rapidly progressive renal failure, defined as a doubling of serum creatinine (SC), reaching a level > 2.5 mg/dL in less than two weeks, with a median survival of two weeks. The best treatment for type 1 HRS is orthotropic liver transplantation (OLT).6 Type 2 HRS is characterized by a moderate and steady reduction in renal function (SC < 2.5 mg/dL). The median survival of patients with type 2 HRS is six months. The management of HRS has focused on improving renal function, thus extending the patients’ survival and allowing the performance of OLT.7 The presence of HRS at the time of OLT is associated with increased morbidity.8
The first trial to show that HRS is reversible was reported by Guevara et al.,9 who evaluated renal function in 16 patients with type 1 HRS. When these patients received ornipressin with albumin, an improvement in renal function was observed at day 15.
Two recently published controlled clinical trials10,11 showed that the administration of terlipressin plus albumin is an effective treatment for the reversal of type 1 HRS (in 43% and 34% of patients). Experience with terlipressin for the treatment of HRS in Mexico is limited. Therefore, this study was conducted to evaluate the effectiveness of an infusion of terlipressin plus albumin in reverting type 1 HRS, its tolerability, and its adverse effects.
Experimental ProceduresThis was a prospective open-label pilot study of the treatment of patients with type 1 HRS with terlipressin plus albumin. Thirteen consecutive patients with cirrhosis and type 1 HRS, diagnosed between January 2006 and August 2008 at the “Dr. José E. Gonzalez” University Hospital, were included in the study. The study was approved by the local Ethical Committee, and relatives gave their written informed consent to the patients’ participation. The disease etiologies were alcohol (n = 4), hepatitis C virus (n = 2), nonalcoholic steatohepatitis (n = 2), autoimmune hepatitis (n = 3), drugs (n = 1), and cryptogenesis (n = 1). All patients received terlipressin (Glyverase, Ferring Pharmaceuticals, Mexico) plus albumin as the treatment for type 1 HRS. The patients were divided in two groups: eight responders (R), in whom type 1 HRS was reversed with treatment; and five nonresponders (NR), in whom type 1 HRS was not reversed with treatment. The diagnosis of type 1 HRS was made according to the IAC criteria.5 The study protocol included monitoring the patients for 24-48 h. Albumin was administered at a dose of 30-80 g/day, and if no renal function improvement was observed, terlipressin therapy was initiated, this was done according the IAC criteria. If patients exhibited hypotension, a dopamine infusion (3-5 μg/kg per minute) was added, and if there was oliguria, furosemide was administered. If no improvement was observed in SC, median arterial pressure (MAP), serum sodium (SS), and urinary sodium (US) 48 h after treatment, terlipressin was commenced at a dose of 0.5 mg i.v. every 4 h, and if there was no reduction in SC, the dose was doubled to > 1 mg every 24-72 h and then up to 2 mg every 4 h. Albumin was continued at the same dose while terlipressin was added. The maximum duration of treatment with terlipressin was 18 days. If no response was observed, treatment with terlipressin was stopped after a maximum of nine days. Treatment was successful when SC decreased to ≤ 1.5 mg/dL, or there was a reduction of at least 20% from the basal SC level. Because one patient developed nonfatal myocardial infarction and pulmonary edema during treatment with terlipressin and albumin, a cardiac echocardiography evaluation before treatment administration was added to the protocol.
Statistical AnalysisThe results are expressed as means ± standard errors of the means. The statistical significance of differences was assessed with the Wilcoxon test for intragroup comparisons and the Mann-Whitney test for analyses between the two groups. A P value of < 0.05 was considered significant.
ResultsAt baseline, there were no differences between the groups in age, sex (Table 1), SS, US, SC, or MAP; nor were there differences in the Child-Pugh classification or model for end-stage liver disease (MELD) scores (Table 2). The evolution time (months) of cirrhosis was longer in patients in the R group than the patients in the NR group (P = 0.03; Table 1). The patients presented diverse comorbidities. In the R group, two patients had type 2 diabetes mellitus (DM), one was obese, one had high blood pressure, and one patient had seizures; within the NR group, two patients had a diagnosis of type 2 DM and one patient was obese. HRS reversal was observed in eight (61%) of the 13 patients who received treatment with terlipressin plus albumin.
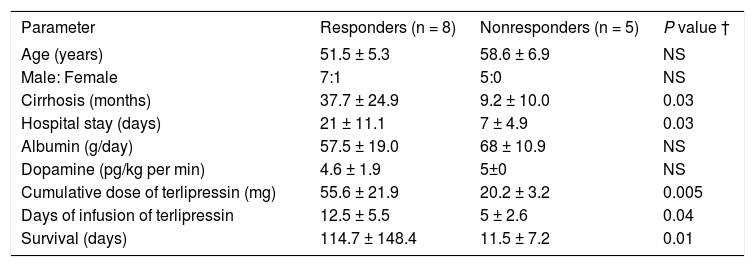
Characteristics of patients with type 1 HRS, treatment, and survival.
| Parameter | Responders (n = 8) | Nonresponders (n = 5) | P value † |
|---|---|---|---|
| Age (years) | 51.5 ± 5.3 | 58.6 ± 6.9 | NS |
| Male: Female | 7:1 | 5:0 | NS |
| Cirrhosis (months) | 37.7 ± 24.9 | 9.2 ± 10.0 | 0.03 |
| Hospital stay (days) | 21 ± 11.1 | 7 ± 4.9 | 0.03 |
| Albumin (g/day) | 57.5 ± 19.0 | 68 ± 10.9 | NS |
| Dopamine (pg/kg per min) | 4.6 ± 1.9 | 5±0 | NS |
| Cumulative dose of terlipressin (mg) | 55.6 ± 21.9 | 20.2 ± 3.2 | 0.005 |
| Days of infusion of terlipressin | 12.5 ± 5.5 | 5 ± 2.6 | 0.04 |
| Survival (days) | 114.7 ± 148.4 | 11.5 ± 7.2 | 0.01 |
NS: Not significant. †: p value, compared between responders and nonresponders.
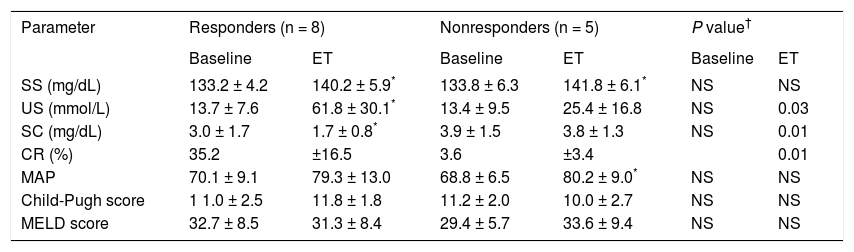
Effects of treatment on renal function and hemodynamics.
| Parameter | Responders (n = 8) | Nonresponders (n = 5) | P value† | |||
|---|---|---|---|---|---|---|
| Baseline | ET | Baseline | ET | Baseline | ET | |
| SS (mg/dL) | 133.2 ± 4.2 | 140.2 ± 5.9* | 133.8 ± 6.3 | 141.8 ± 6.1* | NS | NS |
| US (mmol/L) | 13.7 ± 7.6 | 61.8 ± 30.1* | 13.4 ± 9.5 | 25.4 ± 16.8 | NS | 0.03 |
| SC (mg/dL) | 3.0 ± 1.7 | 1.7 ± 0.8* | 3.9 ± 1.5 | 3.8 ± 1.3 | NS | 0.01 |
| CR (%) | 35.2 | ±16.5 | 3.6 | ±3.4 | 0.01 | |
| MAP | 70.1 ± 9.1 | 79.3 ± 13.0 | 68.8 ± 6.5 | 80.2 ± 9.0* | NS | NS |
| Child-Pugh score | 1 1.0 ± 2.5 | 11.8 ± 1.8 | 11.2 ± 2.0 | 10.0 ± 2.7 | NS | NS |
| MELD score | 32.7 ± 8.5 | 31.3 ± 8.4 | 29.4 ± 5.7 | 33.6 ± 9.4 | NS | NS |
ET: end of treatment. SS: serum sodium. US: urinary sodium. SC: serum creatinine. CR: creatinine reduction. MAP: median arterial pressure. NS: not significant. *P > 0.05 in intragroup comparison. †: p value, compared between groups.
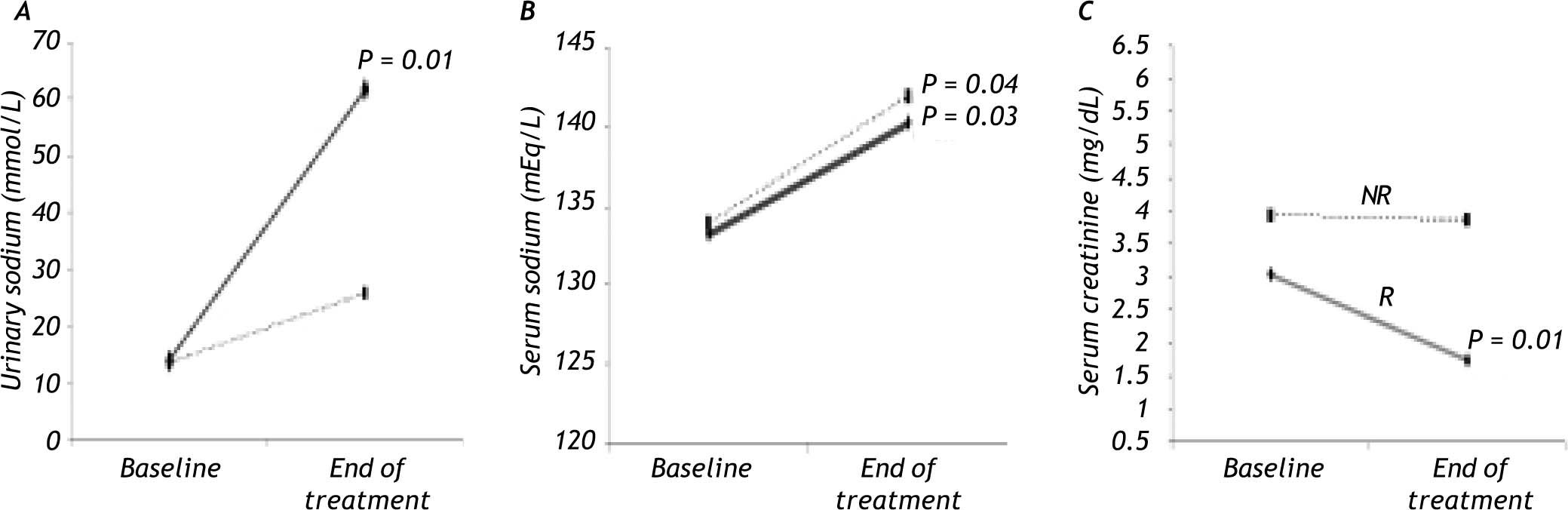
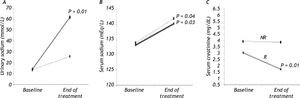
In the R group, SC decreased by 35% (P = 0.01) and there was an increase in SS (P = 0.03) and US (P = 0.01; Figure 1), whereas there was no significant change in MAP during treatment. The Child-Pugh classification and MELD scores did not change during treatment in this group of patients (Table 2).
The NR group showed an increase in SS (P = 0.04) and MAP (P = 0.04) compared with baseline (Figure 1), but no significant change in SC was observed. As in the R group, the Child-Pugh classification and MELD scores remained unchanged (Table 2).
In the R group, one patient presented with pneumonia, one patient presented with upper-gastrointestinal bleeding (UGIB), and another one presented with spontaneous bacterial peritonitis (SBP) as the triggering factors. In the NR group, one patient presented with UGIB.
Some complications were observed during treatment in this study. Three patients in the R group presented with UGIB, and SBP was observed in two patients in the R group.
Patients who responded to treatment were in hospital for a longer period (P = 0.03). The accumulated dose of terlipressin (P = 0.005) and the days of terlipressin infusion (P = 0.04) were higher in the R group than in the NR group (Table 1).
At the follow-up of the R group, one male patient had received OLT two weeks after HRS reversal; one male patient survived for 15 months after the reversal of HRS, with no recurrence; one female patient survived 10 months with no recurrence of HRS, and is still alive; two male patients died of liver failure on the waiting list for OLT, one and two months after HRS reversal; one male patient survived and was lost to follow-up; and the last two patients died less than two weeks after the diagnosis of type 1 HRS. The five patients in the NR group died within three weeks of admission. In two patients, treatment with terlipressin had to be stopped because of adverse events: one developed pulmonary edema and tachycardia on the second day of terlipressin infusion and was classified as a nonresponder because his SC decreased by only 7%, and the other one developed a nonfatal myocardial infarction and pulmonary edema seven days after terlipressin infusion. The SC of this patient had decreased by 57%, so he was classified as a responder. No other ischemic complications were reported. Other adverse effects observed, which did not justify the suspension of treatment, were abdominal pain in two patients (one in each group) and tachycardia in one patient in the R group.
DiscussionThis is the first prospective open-label pilot study reported in Mexico of the treatment of patients with cirrhosis and type 1 HRS with terlipressin plus albumin. The efficacy of terlipressin for HRS in clinical practice is limited for several reasons. The factors predicting the renal response to the drug remain unclear and the rate of adverse effects necessitating the interruption of treatment has not yet been assessed.12
Diverse studies have shown that the administration of terlipressin plus albumin improves glomerular filtration and MAP in patients with type 1 HRS13-16 and in patients with cirrhosis with ascites but without HRS.17-19 In this study, there was a nonsignificant improvement in MAP, unlike other studies.13-15 Hyponatremia has a prevalence of 21.6%20 in cirrhotic patients, and in this study, six patients (46%), four in the R group and two in the NR group, presented with hyponatremia at baseline. At the end of treatment, both the R and NR groups showed an increase in SS to the normal range (P = 0.03 and P = 0.04, respectively). This verifies that hemodynamic function improves in patients treated with terlipressin plus albumin. There was a significant improvement in US in the R group (P = 0.01).
The reversal of HRS with terlipressin plus albumin has been shown in 34%10 and 77%6 of patients. In this study, 61% of patients were classified as responders, and other studies have reported similar figures.13,21,22
At present, the dose and duration of terlipressin treatment for HRS has not been standardized.12 The mean duration of treatment with terlipressin plus albumin has been reported as 2-26 days.12 In our study, the median duration of therapy in the R group patients was 12.5 ± 5.5 days.
Treatment for shorter periods of time improves circulatory function (suppression of plasma renin activity and noradrenaline concentrations) but not renal function.23 Solanki et al.14 used a dose of 1 mg/12 h, Sanyal et al.10 administered 1 mg/6 h, and Martin-Llahí et al.11 administered 1-2 mg/4 h, whereas in this study, the patients received 0.5-2 mg/4 h. In all these studies, the patients showed a favorable response, and in the present study, the reversal rate of type 1 HRS was 61%.
Although the mortality of patients with HRS is very high, the death rate in HRS patients treated with terlipressin is still controversial.12 Previously, we treated a small group of four patients with cirrhosis and type 1 HRS with albumin and dopamine. One patient had a spontaneous reversal of HRS, lived for 10 months, and then was lost to follow-up. The rest died within 19 days of admission, without reversal of HRS.
The incidence of ischemic adverse effects requiring terlipressin discontinuation is approximately 13%.24 Similarly, in our study two patients (15.3%) required treatment discontinuation, one patient presented with ischemic complications (nonfatal myocardial infarction) and pulmonary edema, and another patient developed pulmonary edema.
The range of survival times reported in some studies of type 1 HRS patients treated with terlipressin is 8.5-40 days.13,14 In this study, the survival of the R-group patients was 112.8 days, in contrast with the NR-group patients, whose survival was 11 days (P = 0.01). Some studies have reported the recurrence of HRS after reversal.13,21 We observed no recurrence.
The factors most commonly triggering type 1 HRS include bacterial infections, like SBP, high-volume paracentesis without plasma expansion, and UGIB.24-26 In this study, the triggering factors observed were two bacterial infections and two UGIB. Prevention and early treatment of HRS should avoid or minimize further deterioration of the liver and circulatory functions and renal hypoperfusion. Precipitating factors should also be eliminated, such as the administration of potentially nephrotoxic drugs, the unrestricted use of diuretics, infections, and portal hypertensive bleeding.27
In conclusion, the patients in this study had similar demographic data at baseline and presented multiple comorbidities. The reversal of type 1 HRS was seen in 61% of cirrhotic patients treated with terli-pressin plus albumin. Survival after the reversal of HRS was 112 days. No relapse of HRS was seen. Because adverse effects occur in patients treated with terlipressin, a cardiovascular evaluation is required before the commencement of treatment. Terlipressin appears to be an effective treatment for improving renal function in patients with type 1 HRS, but this must be confirmed by larger controlled trials.
AcknowledgmentsFerring Laboratories of Mexico donated part of the terlipressin used in this protocol.
Abbreviations- •
HRS: Hepatorenal syndrome
- •
IAC: International Ascites Club
- •
OLT: Orthotopic liver transplantation
- •
R: Responders
- •
NR: Nonresponders
- •
SC: Serum creatinine
- •
MAP: Median arterial pressure
- •
SS: Serum sodium
- •
US: Urinary sodium
- •
MELD: Model for End-stage Liver Disease
- •
DM: Diabetes mellitus
- •
UGIB: Upper-gastrointestinal bleeding
- •
SBP: Spontaneous bacterial peritonitis