In the 1980s, Carpentier and Cosgrove, reported the first series of aortic valve repair using Cabrol commissural annulopastia for annular reduction and leaflet wedge resection for prolapse correction. Later T. David and J. Schaefers describes valve-sparing techniques in aortic root surgery for aneurysms. The advantages of valve repair are multiple and also affect survival compared to prostheses. Studies with CT scan of the aortic root and in patients undergoing successful bicuspid valve repair using sub-commissural annuloplasty and Schaefers’ leaflet reconstruction, shown an elliptical configuration of the aortic ring. This has led to the development of a rigid internal ring for the aortic valve with the knowledge obtained with the principles of mitral valve repair. This manuscript describes the bases of the development, principles, implantation technique and current experience of the so-called “internal remodeling rings” for aortic valve repair.
En los años 80, Carpentier y Cosgrove reportaron las primeras series de reparación valvular aórtica usando la anuloplastia comisural de Cabrol y la resección en cuña de velos para corregir la dilatación anular y los prolapsos. Posteriormente, T. David y J. Schaefers describen las técnicas de preservación valvular en la cirugía de la raíz aórtica en caso de aneurismas. Las ventajas de la reparación valvular son múltiples y afectan tambien la supervivencia en comparativa con las prótesis. Estudios con angio-TAC de la raíz aórtica y en pacientes intervenidos con la anuloplastia subcomisural de Schaefers muestran una configuración elíptica del anillo aórtico. Esto ha llevado a desarrollar un anillo interno rígido para corregir la dilatación del anillo aórtico, con el aprendizaje obtenido con los principios de remodelado en la reparación de la válvula mitral. En el presente manuscrito se describen las bases del desarrollo, los principios, la técnica de implantación y la experiencia actual de los denominados «anillos de remodelado interno» para la reparación valvular aórtica.
Since Carpentier's original “French Correction” lecture in 1983,1 autologous repair of the native mitral and tricuspid valves has become the standard of care. While mitral valve surgery was among the highest risk categories in the 1960s and 1970s, conversion to predominant repair has transformed mitral valve procedures into the safest and most successful operations in cardiac surgery.2 At this point, over 80% of surgeries for degenerative mitral valve disease are repair in the United States,3 and that number exceeds 95% in many practices.4 Valve repair for aortic valve insufficiency (AI), however, has lagged behind, and even in the best US centers, only a fourth of AI cases are repaired.5 Moreover, some categories, such as bicuspid valve repair, have had imprecise applications and higher repair failure rates.6 The goal of our research over the past 15 years was to follow Carpentier's strategy and combine geometric ring annuloplasty with effective leaflet reconstruction techniques to standardize the approach to aortic valve repair.
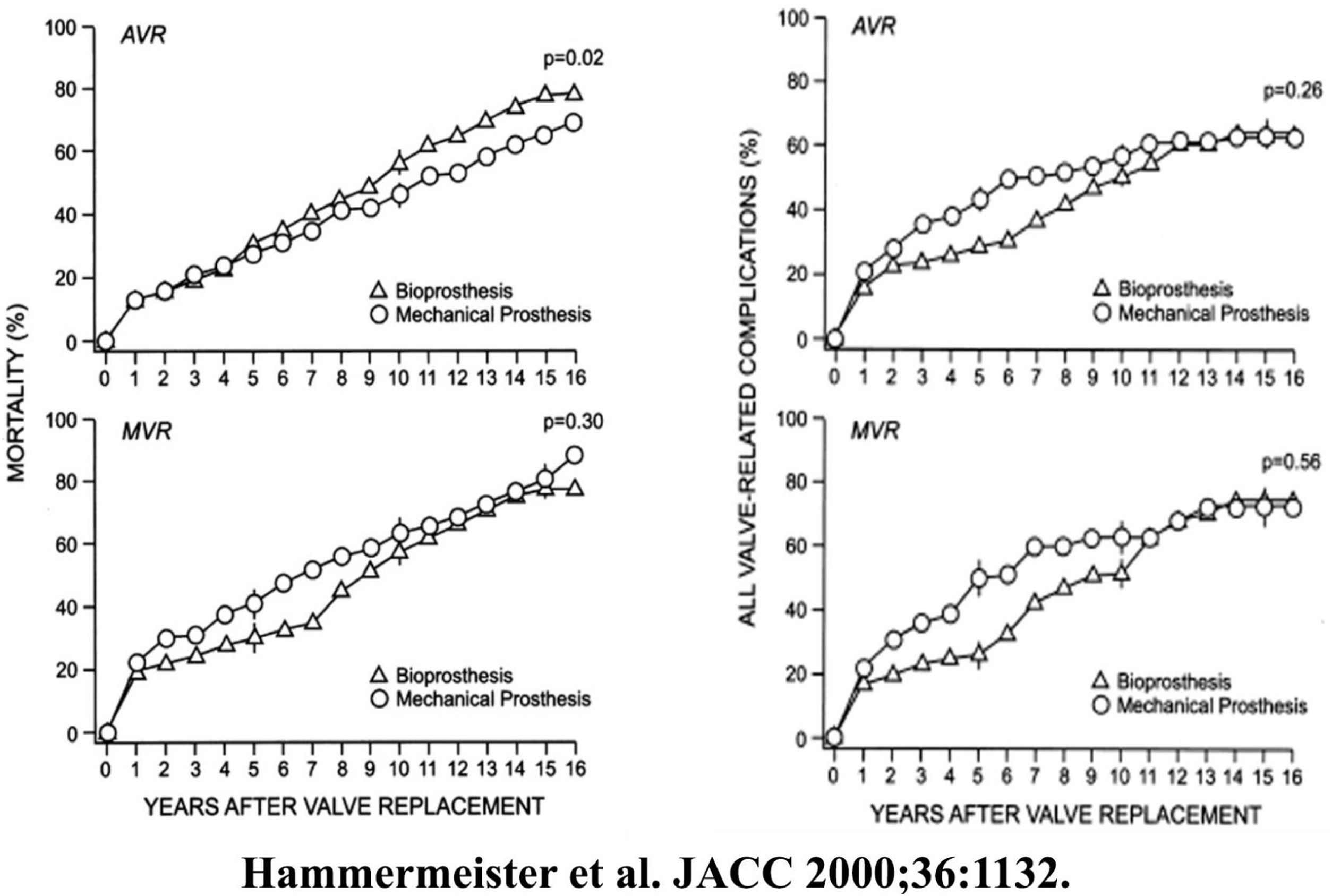
BackgroundIt has been understood for some time that replacing a cardiac valve with a prosthesis only trades one disease for another, with outcomes after prosthetic valve replacement being decidedly suboptimal. In the original VA Cooperative Trial,7 both mechanical and tissue aortic valve replacement in a population of general valve disease was associated with only a 50% survival at 10-years, due to high valve-related complications (Fig. 1). Almost every study since has shown similar results, with survival being equally poor after either mechanical of tissue valve replacement (Fig. 2).8 Moreover, newer tissue valves seem to fare no better (Fig. 3),9 and stentless valves perform similarly. Conversion to autologous valve repair for aortic insufficiency seemed a logical solution.
Left – Cumulative mortality for aortic and mitral valve replacement for bioprostheses and mechanical valves in the VA Cooperative Randomized Valve Trial. Right – Cumulative incidence of valve-related complications for aortic and mitral valve replacement for bioprostheses and mechanical valves in the VA Cooperative Randomized Valve Trial.7
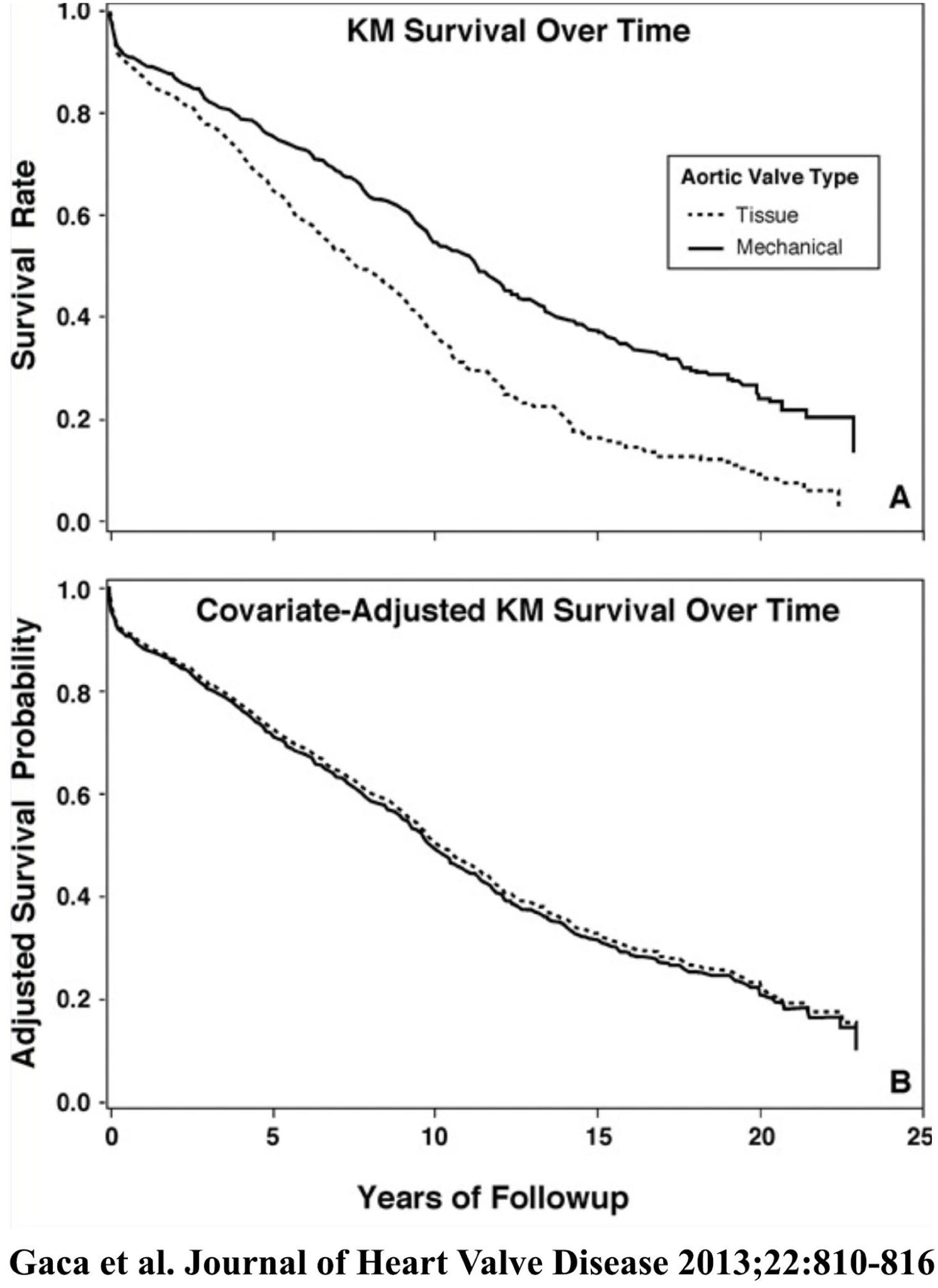
Survival of 2148 patients undergoing aortic valve replacement between 1986 and 2009 in the Duke Cardiovascular Databank (1106 tissue valves and 1040 mechanical valves). Upper panel represents raw survival data, and lower panel is survival comparison after risk adjustment with a Cox proportional hazards model.8
Risk-adjusted survival after bovine pericardial versus porcine aortic valve replacement in Duke Cardiovascular Databank.9 Risk adjustment was performed with a Cox proportional hazards model.
Although Carpentier illustrated aortic valve repair in his 1983 paper,1 it was Cosgrove and associates who developed the first organized program in the late 1980s,10 using Cabrol's commissural annuloplasty11 for annular reduction and leaflet wedge resection for prolapse correction. It soon became evident, however, that repair failure rates with these techniques were unsatisfactory,12 and developmental efforts abated somewhat. During this period, David and associates developed root reimplantation for sparing normal aortic valves during root aneurysm resection,13 and although this approach required strict patient selection, long-term outcomes with autologous aortic valve repair were excellent.14 A recent study confirmed that valve repair using reimplantation reduced late postoperative mortality rate by half (Fig. 4),15 a number that is similar to that observed with the mitral and tricuspid valve surgery.16 Better outcomes, it seems, are due to fewer valve-related complications with valve repair.17,18 The problem, then, was how to develop methods that might achieve the >90% repair rate observed with current methods of mitral repair. Our concept was to follow Carpentier's lead and to develop a geometric annuloplasty ring for the aortic valve.
Risk-adjusted survival after aortic valve repair with valve reimplantation (solid curve) versus prosthetic valve replacement (dashed curve). Risk adjustment was performed with propensity matching.15 Survival with valve repair was similar to the normal age and gender-matched population of Belgium, and the slope of the survival curve (or mortality rate) was reduced by half after valve repair.
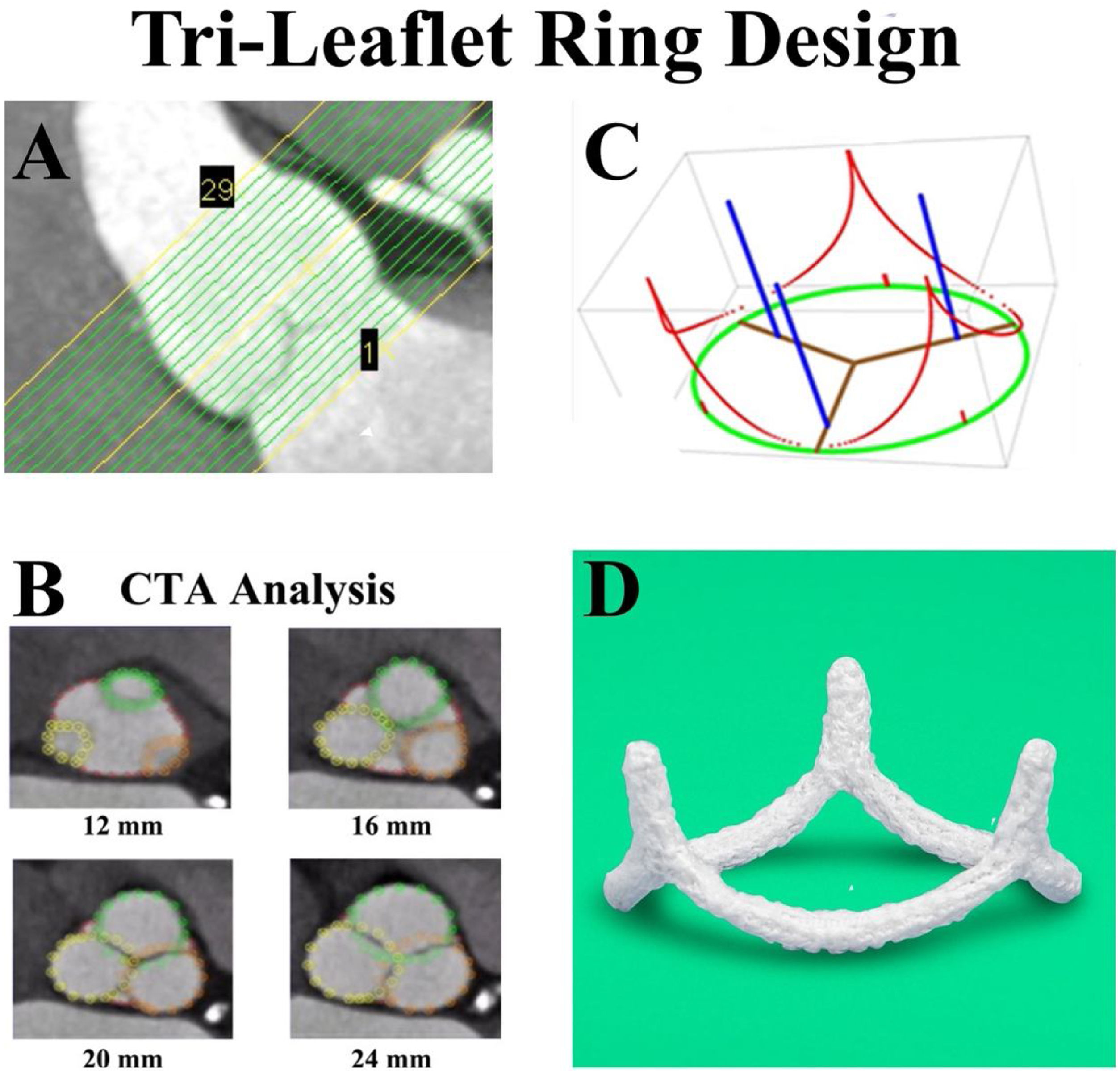
Most advances in medicine involve integration of basic science with clinical care, and aortic valve repair was no exception. The issue was understanding aortic annular geometry better, so that an appropriately shaped ring could be fabricated. After initial studies of human cadaver valves,19,20 high-resolution CT scans were obtained in 11 awake patients undergoing coronary screening with normal aortic valves.21 One millimeter transverse CT slices were made from the left ventricular outflow tract to the sino-tubular junction (Fig. 5A) in 11 awake patients with normal aortic valves undergoing coronary screening. At each transverse cut, high density data points defined each of the root structures, coded for the various leaflets and sinuses (Fig. 5B). Using three-dimensional least squares regression and general ellipsoidal models, the average three-dimensional coordinates for the leaflet-aortic junction were defined (red in Fig. 5C), thus obtaining for the first time the geometry of the aortic valve annulus fibrosus. From these data, a computerized machining device milled annuloplasty rings with similar geometry from solid blocks of Titanium,22,23 covered with a thin layer of polyester to promote endothelialization (Fig. 5D).
Panel A – High-resolution CT angiograms were obtained in 11 awake patients with normal aortic valves undergoing coronary screening. One millimeter transverse cuts were made from the left ventricular outflow tract to above the sino-tubular junction. Panel B – At each vertical level, high density data points defined each of the root structures, coded for the various leaflets and sinuses. Panel C – Using three-dimensional least squares regression and general ellipsoidal models, the average three-dimensional coordinates for the leaflet-aortic junction (red) were defined, thus obtaining for the first time the geometry of the aortic valve annulus fibrosus. Panel D – From these data, a computerized machining device milled annuloplasty rings with similar geometry from solid blocks of Titanium, covered with a thin layer of polyester to promote endothelialization. HAART 300 ring, BioStable Science and Engineering Inc., Austin, TX.
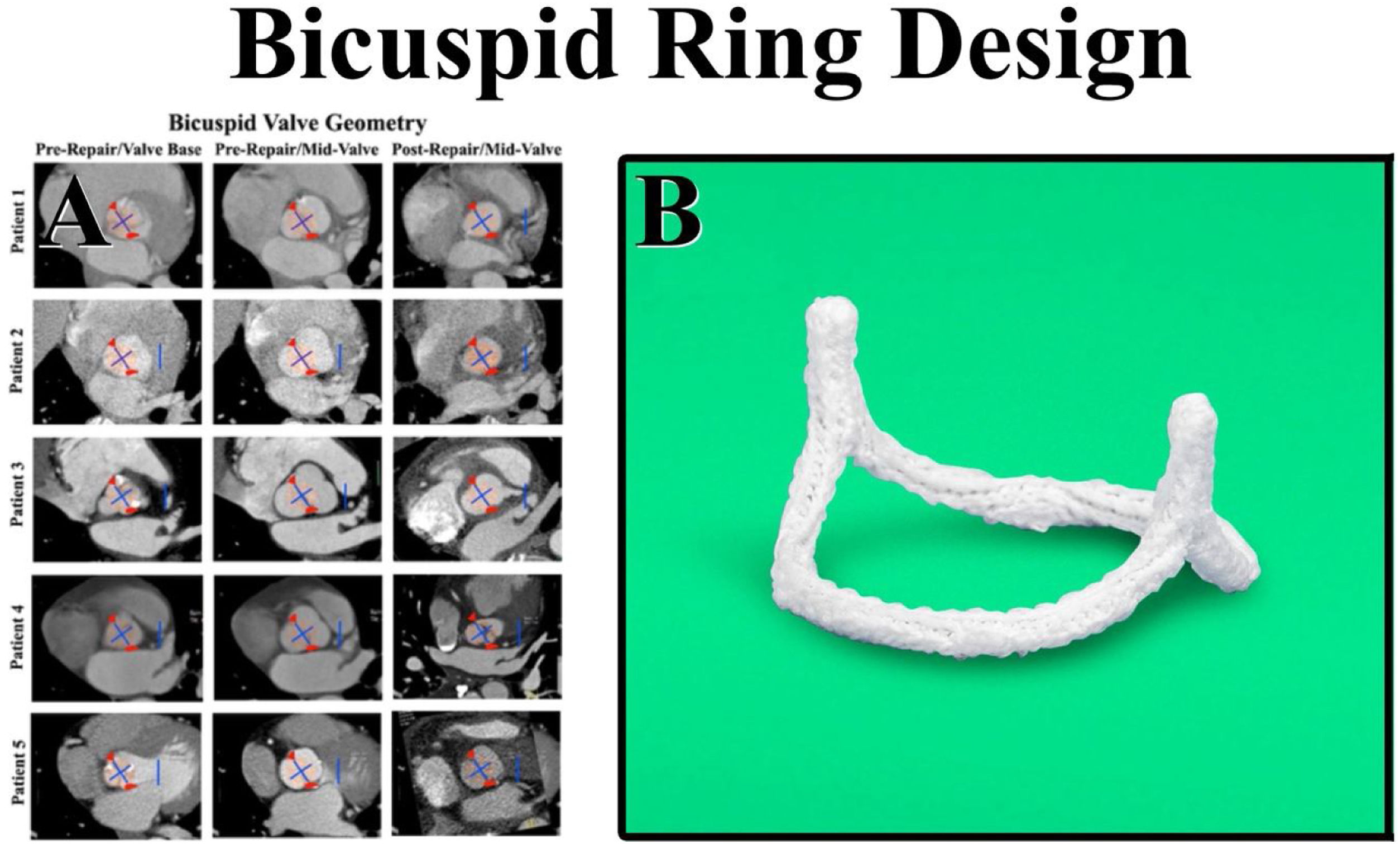
CT angiograms also were obtained in patients undergoing successful bicuspid valve repair using sub-commissural annuloplasty and Schaefers’ leaflet reconstruction.11,24 In Fig. 6, the center column shows a variety of preoperative bicuspid configurations – from a patient with Type 1 right-left leaflet fusion with three equal sinuses to a Type 0 bicuspid valve with equal fused and non-fused sinuses. In all patients, the bicuspid valve was elliptical with the long axis being the sinus-to-sinus diameter. In each preoperative configuration, successful repair seemed to be associated with a more circular base geometry and approximately 180° commissures (Fig. 6). Therefore, sub-commissural post geometry was borrowed from the tri-leaflet ring, and a bicuspid ring with circular base geometry and 180° sub-commissural posts was developed. Each ring was machined from solid blocks of Titanium and covered with a layer of polyester to promote endothelialization.
Panel A – CT angiograms were obtained in patients undergoing successful bicuspid valve repair using sub-commissural annuloplasty and Schaefers’ leaflet reconstruction. The center column shows a variety of preoperative configurations from a patient with Type 1 right-left leaflet fusion and three equal sinuses (Patient 4) – to a Type 0 bicuspid valve with equal fused and non-fused sinuses (Patient 5). In all patients, the bicuspid valve was elliptical with the long axis being the sinus-to-sinus diameter. In all geometries, successful repair was observed to create more of a circular base geometry (pink circles) with approximately 180° commissures (red triangles). Panel B – The sub-commissural post geometry was borrowed from the tri-leaflet ring, and the circular base geometry with 180° commissural posts was machined from solid blocks of Titanium and covered with a layer of polyester to promote endothelialization. HAART 200 ring, BioStable Science and Engineering Inc., Austin, TX.
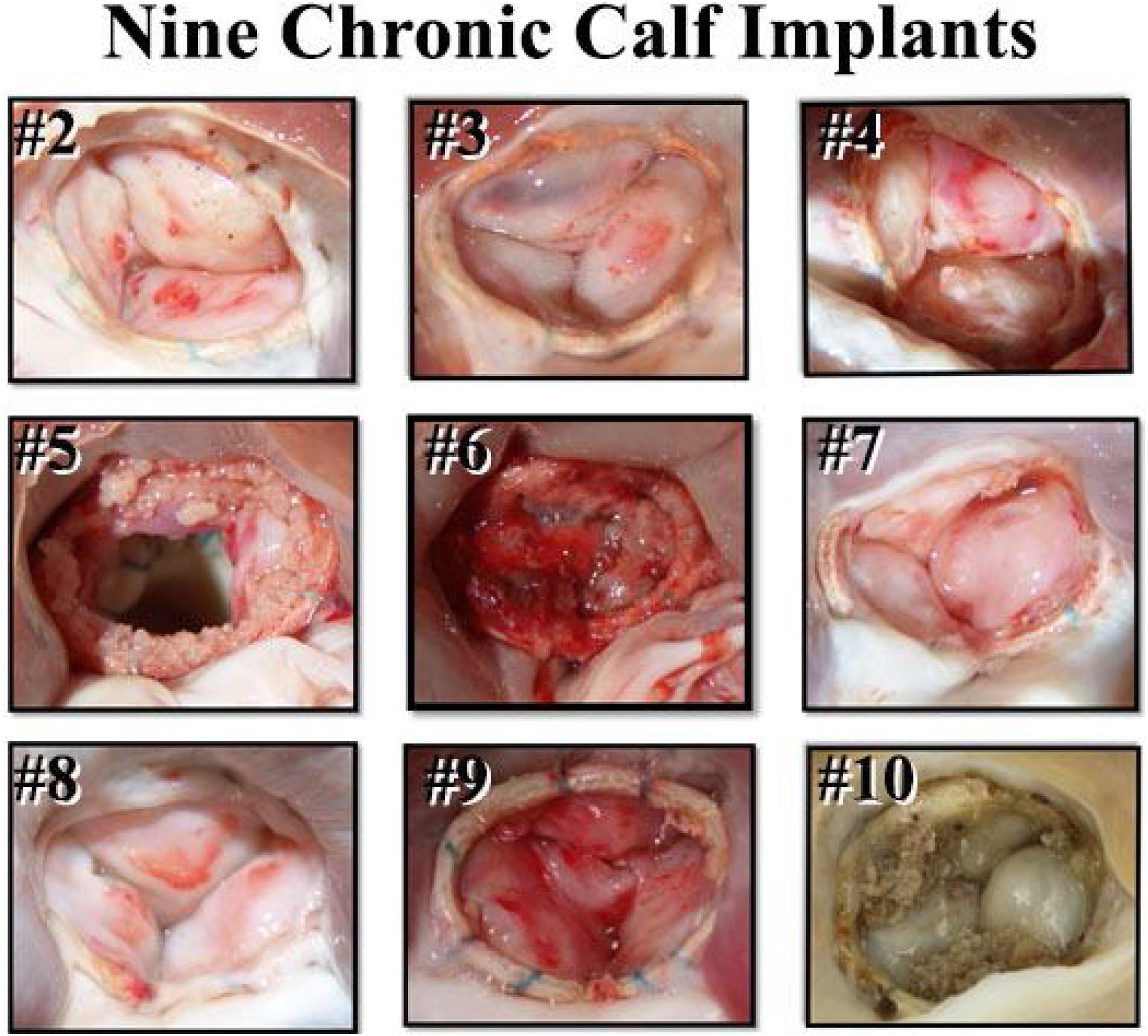
Survival studies then were performed in juvenile calves to test the rings in vivo.25 After 2-month implantations of the tri-leaflet ring, all 9 calves survived and had competent valves (Fig. 7). The favorable appearance of the elliptical ring geometry also was evident. After these successful pre-clinical studies, regulatory trials of both rings were begun in European patients with AI in February 2012,26,27 leading to CE Mark and FDA approval for both devices. Full clinical availability began 5 years later, and to date, over 1400 aortic valve repair cases have been performed using geometric ring annuloplasty. A much better understanding of how to use the device has been developed during the 10-year learning curve, and now, one might suggest that the technology is close to mature. Recent series in medium volume centers, confirmed the reproducibility of this technique with excellent results, making simpler to manage complex aortic repair in centers with low cases with surgeons already experienced in mitral valve repair that follow a systematic approach with this annuloplasty. In subsequent sections, current techniques and outcomes of aortic valve repair using geometric ring annular stabilization will be described.
Post-mortem appearance of 9 calf aortic valves after 2-months of ring implants. All animals survived, and all valves were competent. The leaflet excrescences were determined to be post-mortem clot in animals who were in cold storage for a period before autopsy.25
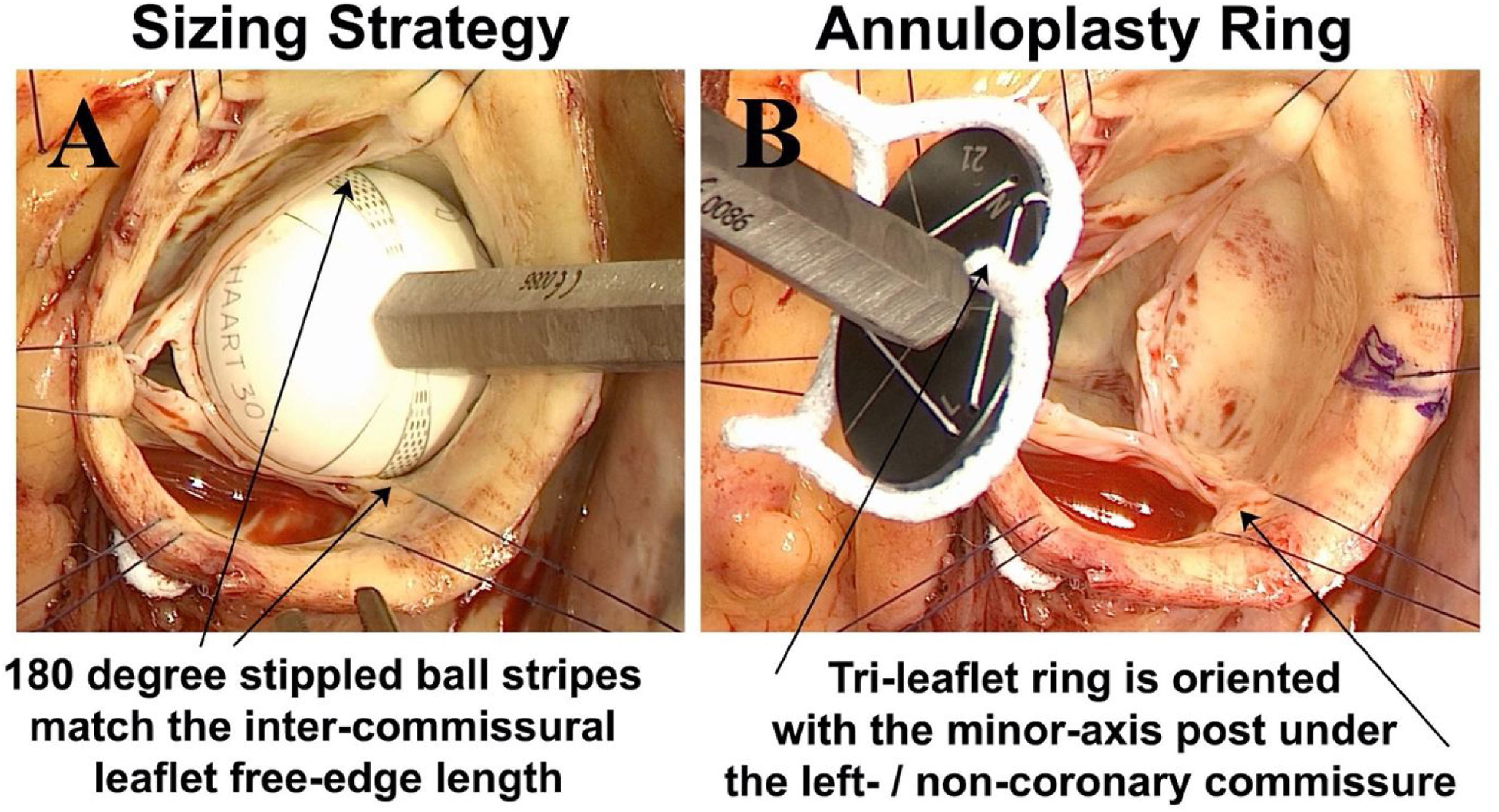
It is now clear that most patients with chronic AI, either with tri-leaflet or bicuspid valves, have significant annular dilatation, averaging around 5mm larger than what leaflet size would predict. In important human observations, normal aortic annular diameter averaged around 21–22mm,28 whereas in our clinical studies of pathologic annuli, both in tri-leaflet and bicuspid valves, average annular diameter was quite dilated at 26–27mm.29,30 Moreover, annular diameter required for competence did not correlate well with patient size or other demographic variables, so that predicting the degree of appropriate annular reduction is problematic with existing approaches. As described in more detail elsewhere,31 annuloplasty ring size is predicted by measuring leaflet free-edge length (FEL) using a special ball sizer (Fig. 8A). Then, FEL/1.5=annuloplasty ring size, as determined in our anatomic studies.20 Thus, the ring reduces and remodels the annulus to match annular diameter to specific leaflet size, providing a fully objective method of annuloplasty sizing. The elliptical rings are positioned so that the minor axis post is sutured beneath the left/non-coronary commissure (Fig. 8B), as in normal valve geometry.
Panel A – Leaflet free-edge length (FEL) is measured from commissure to commissure using special ball sizers positioned in the sinus. FEL/1.5 determines the ring diameter required for valve competence. Panel B – The elliptical tri-leaflet ring is positioned so the minor axis post is posterior – beneath the left/non-coronary commissure, and the right coronary leaflet is opposite on the broad anterior annulus.
Exposure is of utmost importance, and is achieved with six commissural and sinus traction sutures [Video 1: (http://www.jsrmd.com/ftp/330_DARAc.mp4)]. The rings are positioned under the valve annulus using trans-annular horizontal mattress sutures (Fig. 9A), taking care to pass the sutures 2mm deep to the leaflet-aortic junction above and below the valve to prevent contact between ring Dacron and leaflet tissue. Initially, a Cabrol-like mattress suture is placed in each commissure to bury the ring posts back into the sub-commissural triangles. Then the ring is passed below the valve, and looping sutures secure each sinus aspect to the corresponding annulus. Small polyester pledgets are positioned supra-annularly, and each suture knot is fixed laterally to prevent contact between suture tails and leaflets (Fig. 9B). The ring posts should be positioned low in the sub-commissural triangle to raise the commissures relative to the base, and increase leaflet vertical coaptation height (Fig. 9B). For best results, 3-0 coated braided suture should be used (e.g. Tycron, Teleflex Medical, Wayne, PA), along with a 20-mm half circle taper needle. The larger needle makes the looping sutures easier, and braided suture has performed better with less suture fracture than Prolene.30
Panel A – The ring posts are first sutured beneath the sub-commissural triangles using Cabrol-like horizontal mattress sutures, and the device is lowered below the valve. Then, trans-annular horizontal mattress looping sutures fix the ring under the annulus, taking care to leave no gaps or loose sutures. Panel B – After repair, the lateral fixation sutures are evident to direct suture tails laterally away from the leaflets. With low positioning of the ring posts low in the sub-commissural triangle, the leaflets become more vertical, augmenting leaflet coaptation height.
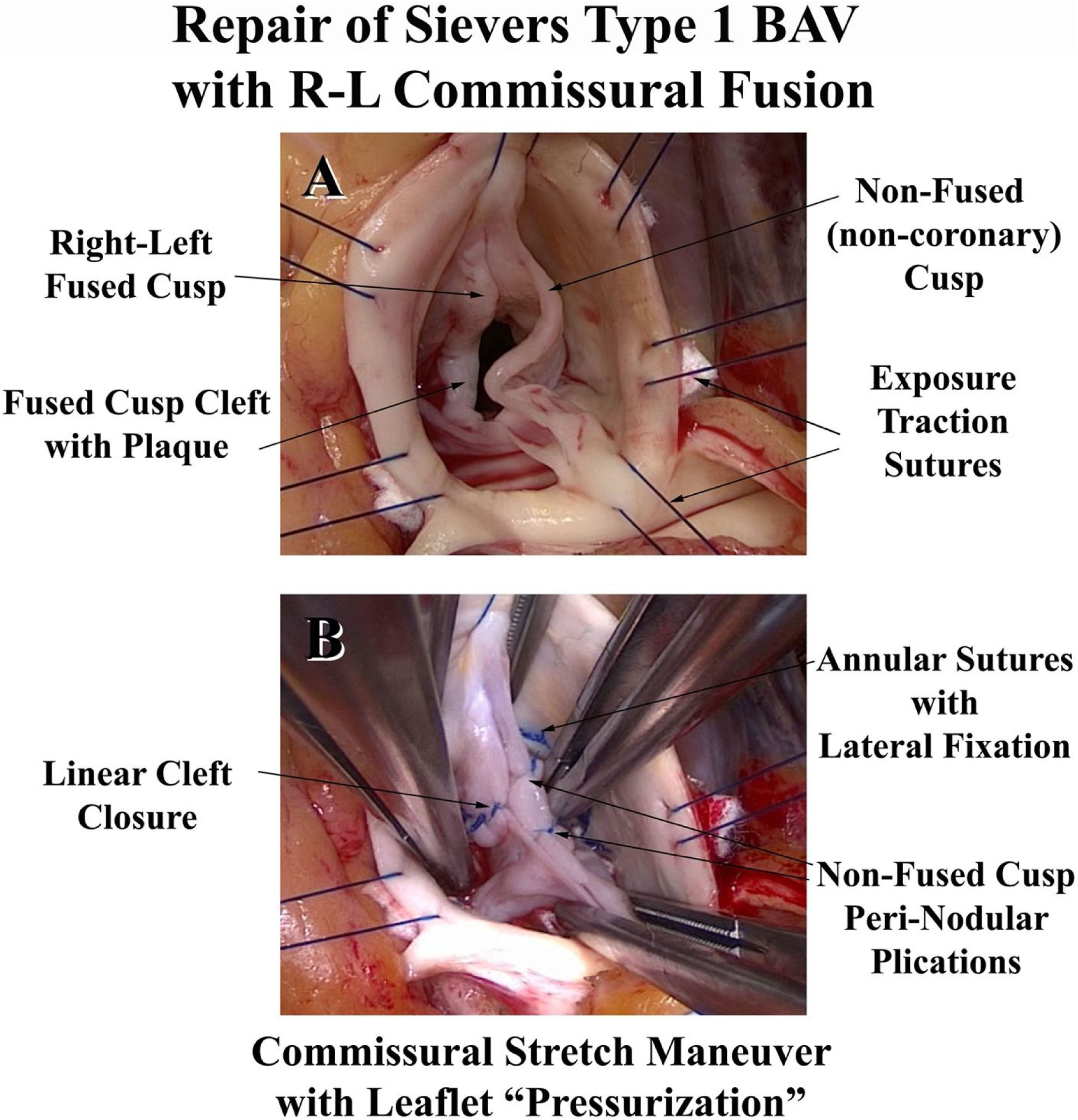
Most patients with significant AI have some type of leaflet defect.26 Leaflet prolapse is the most common, followed by retraction and scarring of the Nodulus Arantius. Ruptured fenestrations or commissures can induce prolapse. Knowledge of leaflet repair techniques, such as leaflet plication, or nodular release using the ultrasonic aspirator (Fig. 10), is critical, and is provided in detail elsewhere.31,32 Aortic ring annuloplasty is especially useful for bicuspid and unicuspid valve repair, since it produces major remodeling of the annulus into 180° commissural geometry with equal fused and non-fused annuli (Fig. 11). Schaefers’ leaflet reconstruction techniques24 are combined with ring annuloplasty [Video 2: (http://www.jsrmd.com/ftp/353_TBAVc.mp4)], allowing the full spectrum of BAV defects, including unicuspid valves,33 to be repaired.27,30,31 The technology has been applied to other congenital lesions, such as dilated Ross autografts or Transposition/Switch valves, and the results have been good.34 In each of these disorders, specific aneurysmal sinus segments are resected and selectively replaced with tongues of a remodeling graft.31,35 For any proximal aortic replacement, a graft is selected at 7-mm larger than ring diameter, consistent with normal root geometry.22,23 Finally, aortic ring annuloplasty is useful in patients undergoing multiple valve repair, and techniques are available for most lesions creating AI.36 As a routine, only aspirin anticoagulation is employed in patients without atrial fibrillation.
Panel A – A prolapsing Type 0 bicuspid valve is undergoing free-edge plication with simple 6-0 Prolene sutures, passed into the leaflet free-edge chord. Panel B – Shortening of the free-edge raises the leaflet and corrects the prolapse. Panel C – A scarred retracted Nodulus Arantius is thinned with the ultrasonic aspirator to improve Nodular suppleness and central leaflet coaptation.
Features in the repair of a Type 1 right-left commissural fusion bicuspid valve. Panel A shows the right-left commissural fusion cleft along with a prolapsing non-coronary cusp. Panel B illustrates the repair features with annuloplasty ring insertion, non-fused leaflet plication, and linear cleft closure. The leaflets now have equal free-edge lengths and coaptation heights.
Because of superior outcomes, aortic valve repair is now the treatment of choice for many forms of AI.37–39 Compared to prosthetic valve replacement, aortic valve repair exhibits lower risk-adjusted operative mortality40 and fewer long-term valve-related complications.17,18 Repair with geometric ring annuloplasty is no exception, with operative mortalities of less than 1% and rare valve-related complications.30,41,42 The issue with any valve repair is reducing rates of repair failure to a minimum. All new techniques have experienced a learning curve, including valve reimplantation in the 1990s,43,44 root remodeling in the 2000s,17 and geometric ring annuloplasty in the last decade. In the first clinical trials of the annuloplasty ring starting 10-years ago, the 2-year repair failure rate was 11%.26 Causes were related primarily to annular sutures coming untied and leaflet lacerations from long annular suture tails. The “lateral suture fixation” technique was developed45 that largely eliminated these causes of repair failure. After institution of this technique and clinical introduction of the devices in Europe and the US, and repair failure rates were cut approximately by half.
However, with the larger number of patients treated, other mechanisms of repair failure came to light, producing a persistent 3.5% repair failure rate within 2-years.30 These almost always were related to contact between the annular sutures or ring Dacron and leaflet tissue, producing leaflet abrasion. Many were due to fracture of the Proleneannular sutures used to place the ring, and when the ring was released into the center of the valve, leaflet abrasion resulted. As a consequence, recommendations were changed 1.5 years ago to performing annular suturing with coated braided polyester sutures, and this mode of failure has not been experienced since. The braided Dacron suture needs to be coated with Teflon or Silicone to increase lubricity, since it is important to achieve a tight apposition of the ring and annulus. Similarly, any technical inaccuracy that creates ring-leaflet contact will induce leaflet abrasion and failure, such as (1) suturing the ring into the base of the leaflet (sutures need to be placed 2-mm deep to the leaflet-aortic junction above and below the valve), (2) loose sutures or suture gaps that allow movement of the ring under the leaflet, and (3) any contact between annular suture tails and leaflet tissue. Annular suture technique is not difficult, but it does require precision to avoid complications.
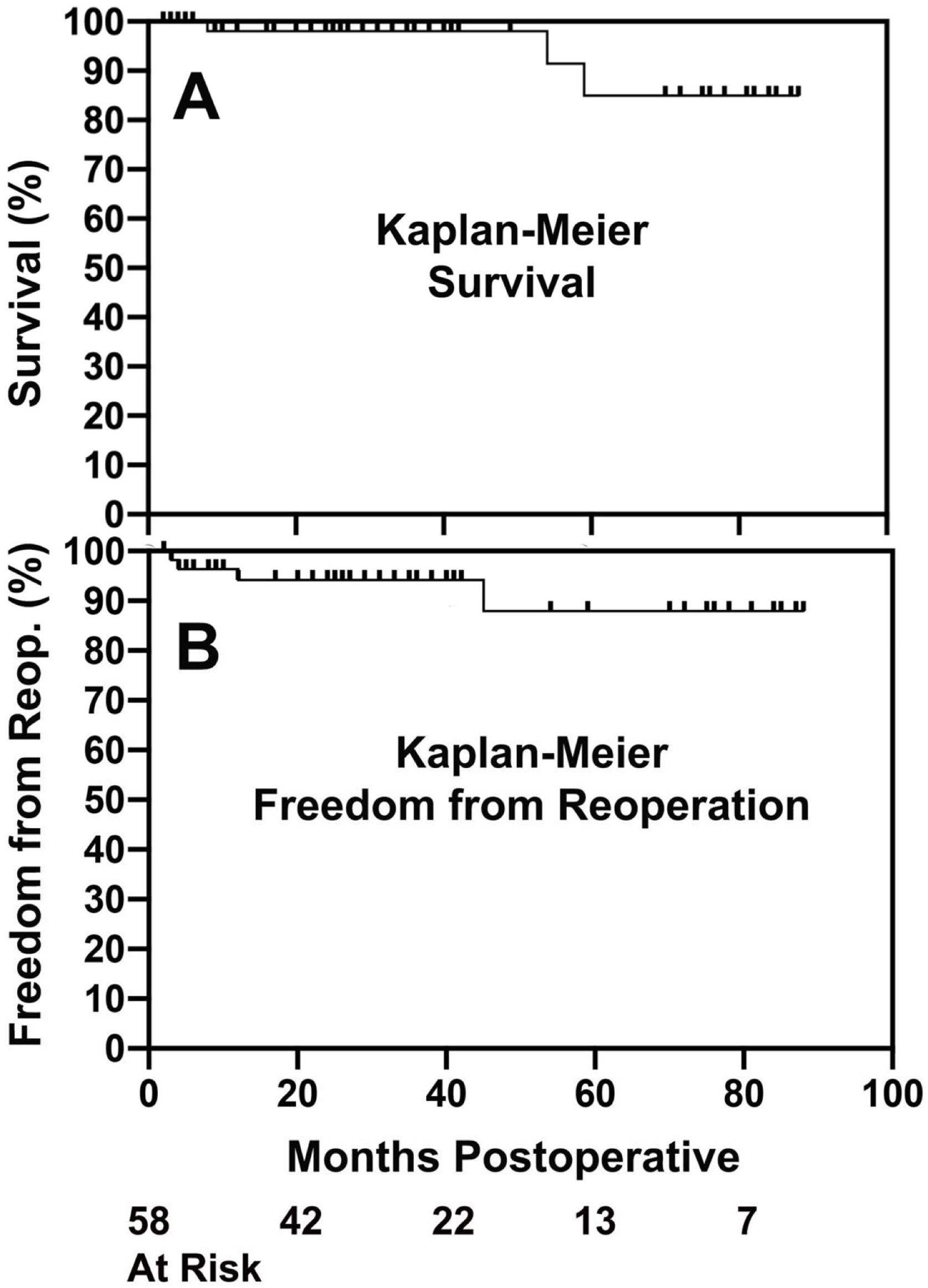
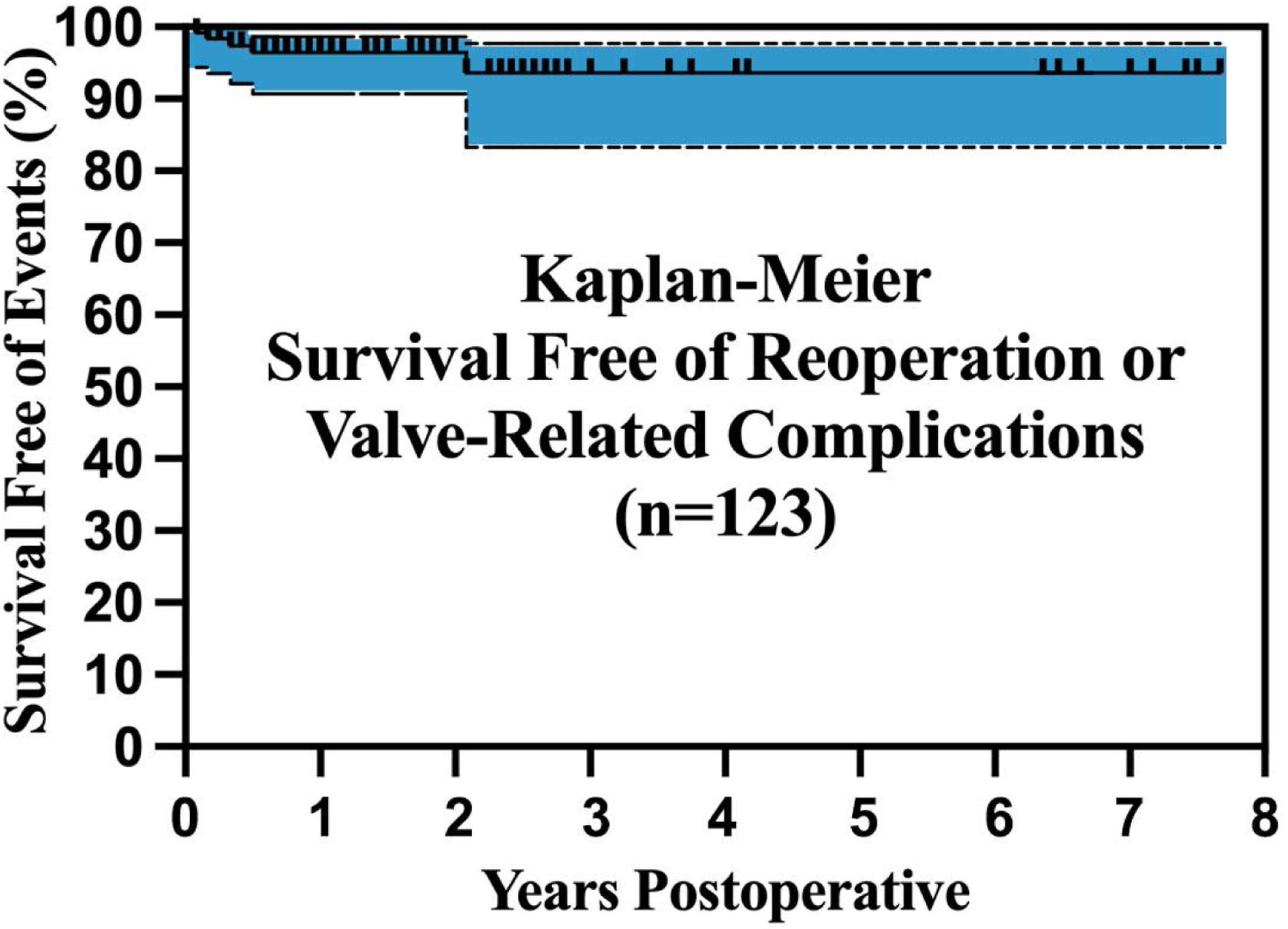
Initial regulatory trials of tri-leaflet and bicuspid ring annuloplasty26,41,45 were continued to 2-years of follow-up and showed good results. Operative mortality and morbidity were low, AI reduction was good, mean valve gradients were acceptable, and survival free of complications was excellent. Longer-term followup is now becoming available, and these trends continue. With tri-leaflet ring annuloplasty, survival free of reoperation exceeded 80% at 88 months of follow-up, despite the learning curve repair failures described above (Fig. 12).42 The outcomes with bicuspid repair were similar (Fig. 13), with survival free of reoperation or valve-related complications exceeding 80% at almost 8-years.30 Moreover, these analyses included the small incidence of technical failures described above, and the potential exists for outcomes to be even better with increasing experience. These data confirm that geometric ring annuloplasty achieves similar results to other types of AI repair, but with the demonstrated potential for treating 90–95% of AI pathologies.31 It must be emphasized that these approaches are different from previous techniques, and for best results, a period of remote video proctoring is desirable to traverse the learning curve and obtain the best initial outcomes without technical repair failures.
Kaplan–Meier survival (Panel A) and freedom from reoperation (Panel B) after predominant tri-leaflet aortic valve repair with geometric ring annuloplasty.42 Outcomes are consistent with other types of valve repair, except that a wider spectrum of AI pathologies were treated in this series.
Outcomes observed in 123 patient having bicuspid valve repair utilizing geometric ring annuloplasty. The patients with longer follow-up were operated in the original regulatory trials of the BAV ring.27 Again, outcomes are consistent with other types of bicuspid valve repair, except that a wider spectrum of BAV pathologies were managed using ring annuloplasty, including unicuspid valves and very asymmetric sinus geometries.
Aortic valve repair has emerged as a superior alternative for management of patients with AI. Operative risk is low, efficacy currently is satisfactory (and likely to improve further), and late complications are low. Long-term patient survival is augmented. The problem has been the inability of repairing the majority of AI pathologies, but using geometric ring annuloplasty, repair can be extended to 90–95% of AI patients. This approach also allows a standardized approach that is much less subjective and dependent on surgical “artistry”. As such, a new opportunity exists for improving the care of cardiac surgical patients with AI.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestDrs. Rankin and Gerdisch are consultants for BioStable Science and Engineering, Inc, Austin, TX, USA. Dr. Sáez de Ibarra declares receiving honoraria for conferences from Edwards Lifesciences.