Repair of the bicuspid aortic valve has evolved in the past 20 years. The aim of this study was to review two decades of experience and analyze the long-term stability of BAV repair.
MethodsBetween 10/1998 and 12/2020, 547 adult patients (92% male; 41.9±13.4 years) underwent a bicuspid aortic valve repair with (n=400) or without (n=147) annuloplasty. Sinus plication was performed in 175 instances (32%). Mean follow-up of 73.3±56 months (median 59.9 months); it was 92% complete. Survival and freedom from reoperation were calculated.
ResultsSurvival at 20 years was 93.2%±2.7, freedom from reoperation at 20 years was 83.7±2.9. Freedom from AI≥II was 77.7%±3.4 at 15 years. By logistic regression analysis, the use of pericardial patches for cusp repair (p=0.019; OR 2.6), the use of subcommissural plication (p<.0001; OR 7.3), and the lack of annuloplasty (p<.0001; OR 17) were independent predictors for reoperation.
ConclusionIsolated bicuspid aortic valve repair is associated with excellent survival, and the majority of BAV repairs will remain stable over more than 10 years. The use of an annuloplasty improves repair durability and patient survival. Cusp calcification and cusp repair using a pericardial patch are predictors for early valve failure.
La reparación de la válvula aórtica bicúspide ha evolucionado en los últimos 20 años. El objetivo de este estudio fue revisar nuestra experiencia de dos décadas y analizar la estabilidad a largo plazo de la reparación de BAV.
MétodosEntre octubre de 1998 y diciembre de 2020, 547 pacientes adultos (92% varones; 41,9 ± 13,4 años) se sometieron a una reparación de la válvula aórtica bicúspide con (n = 400) o sin (n = 147) anuloplastia. La plicatura de senos se realizó en 175 casos (32%). Seguimiento medio de 73,3 ± 56 meses (mediana 59,9 meses); fue completado en un 92%. Se calculó la supervivencia y la ausencia de reoperación.
ResultadosLa sobrevida a los 20 años fue de 93,2% ± 2,7, la ausencia de reoperación a los 20 años fue de 83,7 ± 2,9. La ausencia de IA ≥ II fue del 77,7% ± 3,4 a los 15 años. Mediante análisis de regresión logística, el uso de parches pericárdicos para la reparación de las cúspides (p = 0,019; OR 2.6), el uso de la plicatura subcomisural (p < 0,0001; OR 7,3), y la falta de anuloplastia (p < 0,0001; OR 17) fueron predictores independientes para la reoperación.
ConclusiónLa reparación aislada de la válvula aórtica bicúspide se asocia con una excelente supervivencia, y la mayoría de las reparaciones de BAV se mantendrán estables durante más de 10 años. El uso de una anuloplastia mejora la durabilidad de la reparación y la supervivencia del paciente. La calcificación de la cúspide y la reparación de la cúspide con un parche pericárdico son predictores de la falla temprana de la válvula.
The bicuspid aortic valve (BAV) is the most frequent congenital cardiac anomaly.1 It is characterized by partial or complete fusion between two cusps and the presence of a nonfused cusp. Prolapse of the fused cusp and annular dilatation are the most common intraoperative findings.2 Patients with a bicuspid aortic valve frequently require surgical intervention for aortic regurgitation (AR) and/or aneurysm at a young age.3
Preservation or repair of the aortic valve has evolved in the past 20 years. First attempts were made at treating isolated aortic regurgitation by repair.4,5 Also operations were developed to treat patients with aortic root aneurysm and presumably normal cusps.6,7 With increasing knowledge of the anatomy of the aortic valve and repair options, both procedures have progressed in the past 20 years.8 Repair leads to a high freedom from valve-related complications and good survival if an adequate valve durability can be achieved.8,9
In the meantime, aortic valve repair has become a routine procedure in experienced centers, and most BAVs can be preserved or repaired.10 Anatomic characteristics of the valves have been defined that require to be addressed, such as commissural orientation, annular dilatation or prolapse.11 Thus, rather than improvising the operation, repair has changed into a systematic approach with clear identification and subsequent correction of the mechanisms leading to regurgitation. In particular, using the concepts of effective height12 and geometric height,13 excellent repair durability and freedom from valve-related complications have been achieved.8,9 Creation of symmetric commissural orientation has improved repair durability further. In addition, we introduced a suture annuloplasty to correct annular dilatation with good midterm results.14
During the past 20 years, we have consistently applied this differentiated anatomic concept in the repair of BAVs. With follow-up reaching more than 20 years, we now intend to analyze the long-term durability of isolated BAV repair.
MethodsPatient populationBetween October 1998 and December 2020, 547 patients with a bicuspid aortic valve underwent isolated repair for AR in our institution. These patients were the subject of this study. The investigation was approved by the respective ethics committee (Saarland Regional Ethics Committee, CEP 202/19), and individual patient consent was waived for the analysis and publication in anonymized fashion.
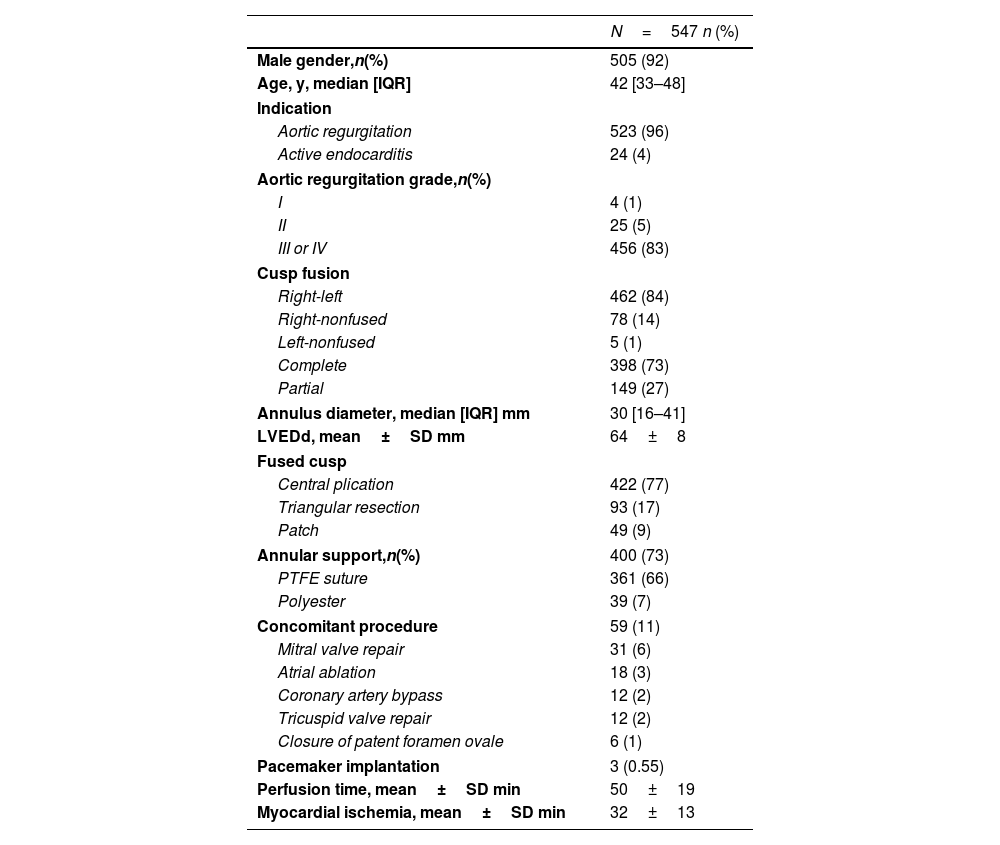
The patient age ranged from 5 to 79 years (mean 41.9±13.4 years). Most of the patients were male (n=507; 92.7%) (Table 1). Preoperative AR was relevant (III° or IV°) in most patients (n=516; 94.33%). The left ventricular end-diastolic diameter ranged from 37 to 84mm (mean 63.73±7.4). The mean sinus diameter was 36.2±4.2mm, and the mean annulus diameter was 30.1±3.5.
Perioperative patient data.
| N=547 n (%) | |
|---|---|
| Male gender,n(%) | 505 (92) |
| Age, y, median [IQR] | 42 [33–48] |
| Indication | |
| Aortic regurgitation | 523 (96) |
| Active endocarditis | 24 (4) |
| Aortic regurgitation grade,n(%) | |
| I | 4 (1) |
| II | 25 (5) |
| III or IV | 456 (83) |
| Cusp fusion | |
| Right-left | 462 (84) |
| Right-nonfused | 78 (14) |
| Left-nonfused | 5 (1) |
| Complete | 398 (73) |
| Partial | 149 (27) |
| Annulus diameter, median [IQR] mm | 30 [16–41] |
| LVEDd, mean±SD mm | 64±8 |
| Fused cusp | |
| Central plication | 422 (77) |
| Triangular resection | 93 (17) |
| Patch | 49 (9) |
| Annular support,n(%) | 400 (73) |
| PTFE suture | 361 (66) |
| Polyester | 39 (7) |
| Concomitant procedure | 59 (11) |
| Mitral valve repair | 31 (6) |
| Atrial ablation | 18 (3) |
| Coronary artery bypass | 12 (2) |
| Tricuspid valve repair | 12 (2) |
| Closure of patent foramen ovale | 6 (1) |
| Pacemaker implantation | 3 (0.55) |
| Perfusion time, mean±SD min | 50±19 |
| Myocardial ischemia, mean±SD min | 32±13 |
SD, standard deviation; IQR, interquartile range; LVEDd, left ventricular end-diastolic diameter; PTFE, polytetrafluoroethylene.
Cusp fusion was mostly seen between the right and the left coronary cusp (n=462; 84.5%) (Table 1). The commissural orientation of the nonfused cusp varied with predominant orientation of 160° or more (n=353; 64.5%). An asymmetric orientation (140–155°) was present in 160 patients (29.3%) and a very asymmetric orientation (<140°) was observed in 51 individuals (9.3%). Limited cusp calcification was present in 47 individuals (8.6%), in most instances in the raphe.
Surgical techniqueThe chest was opened via median sternotomy, and aorta and right atrium were cannulated. The aorta was opened via transverse incision 5–10mm above the sinotubular junction, and blood cardioplegia was given directly into the coronary ostia. The circumferential orientation was determined and maintained using stay sutures placed into the commissures and fixed to the chest wall of the patient. Valve assessment included measurement of both geometric and effective height, the diameter of the basal ring was evaluated by direct intubation using a Hegar dilator.
Repair was performed if the geometric height of the nonfused cusp exceeded 20mm in order to rule out relevant retraction, otherwise valve replacement was performed. Prolapse was defined as an effective height less than 9mm in the nonfused cusp. In the fused cusp, prolapse was determined by visual comparison of the height of the free cusp margin relative to the nonfused cusp.
Prolapse of the fused cusp was corrected by plication of the central free margin. A triangular resection was performed if extreme tissue redundancy, dense fibrosis, or limited calcification made direct tissue adaptation difficult or impossible. In case of more extensive calcification, a patch of autologous pericardium was inserted. The patch was trimmed and inserted into the tissue defect of the fused cusp using continuous polypropylene suture (Prolene 5-0,Ehicon, Hamburg, Germany).
A suture annuloplasty was added to reduce dilatation of the basal ring if it was larger than 26mm. In the first 39 patients, a braided polyester suture was used, in the remaining 361 individuals an expanded polytetrafluorethylene (PTFE) was employed (Gore-Tex CV-0; WL Gore and Associates, Munich, Germany). As described previously (), the annuloplasty suture was either tied around a 21mm, 23mm, or 25mm Hegar dilator according to body surface area (less than versus greater than or equal to 2m2).
Follow-upAll patients were seen regularly by referring cardiologists or the institutional clinic. Echocardiograms or their reports from both institutions and patients’ cardiologists were reviewed.15 In addition, the patients were contacted via phone or seen in clinic to determine current functional status. The cause of death was determined by review of hospital charts or information was sought from respective primary care physicians.
Follow up was 92% complete (3341 patient-years) with a mean follow-up of 73.3±56 months (median 59.9 months). The first time of AI≥2 was recorded for a time-to-event analysis.
Statistical analysisContinuous data are expressed as mean±standard deviation. Data taken from the Kaplan–Meier curve are expressed as mean±standard error. A p value<0.05 was considered statistically significant. Differences between continuous variables were compared by Student's t-test or by Mann–Whitney U test in case of inhomogeneous variances. Categorical data were compared using chi-square test. All data were analyzed using statistical package SPSS version 28 (SPSS Inc., Chicago, IL, USA). In logistic regression analysis, a p value less than 0.10 in the univariable analysis was defined for entry into the multivariable analysis. We applied a stepwise procedure for selecting variables based on the Wald criterion of forward induction. We ended up with the following results.
ResultsConcomitant procedures were performed in 59 patients (Table 1); these included coronary artery bypass grafting (n=12), mitral repair (n=31), tricuspid valve repair (n=12), and left atrial ablation (n=18). Plication of the fused cusp was performed in 422 instances (77.1%).
The duration of myocardial ischemia ranged from 12 to 118min (mean 32±13min). The mean time of extracorporeal circulation time was 49.7±18.7min. Reexploration for hemorrhage was necessary in 7 patients (1.3%). Three patients (0.55%) required pacemaker implantation for postoperative atrioventricular block were necessary.
SurvivalOne patient died early for an in-hospital mortality of 0.18%. The patient underwent surgery for active endocarditis and suffered a perioperative stroke with intracerebral hemorrhage.
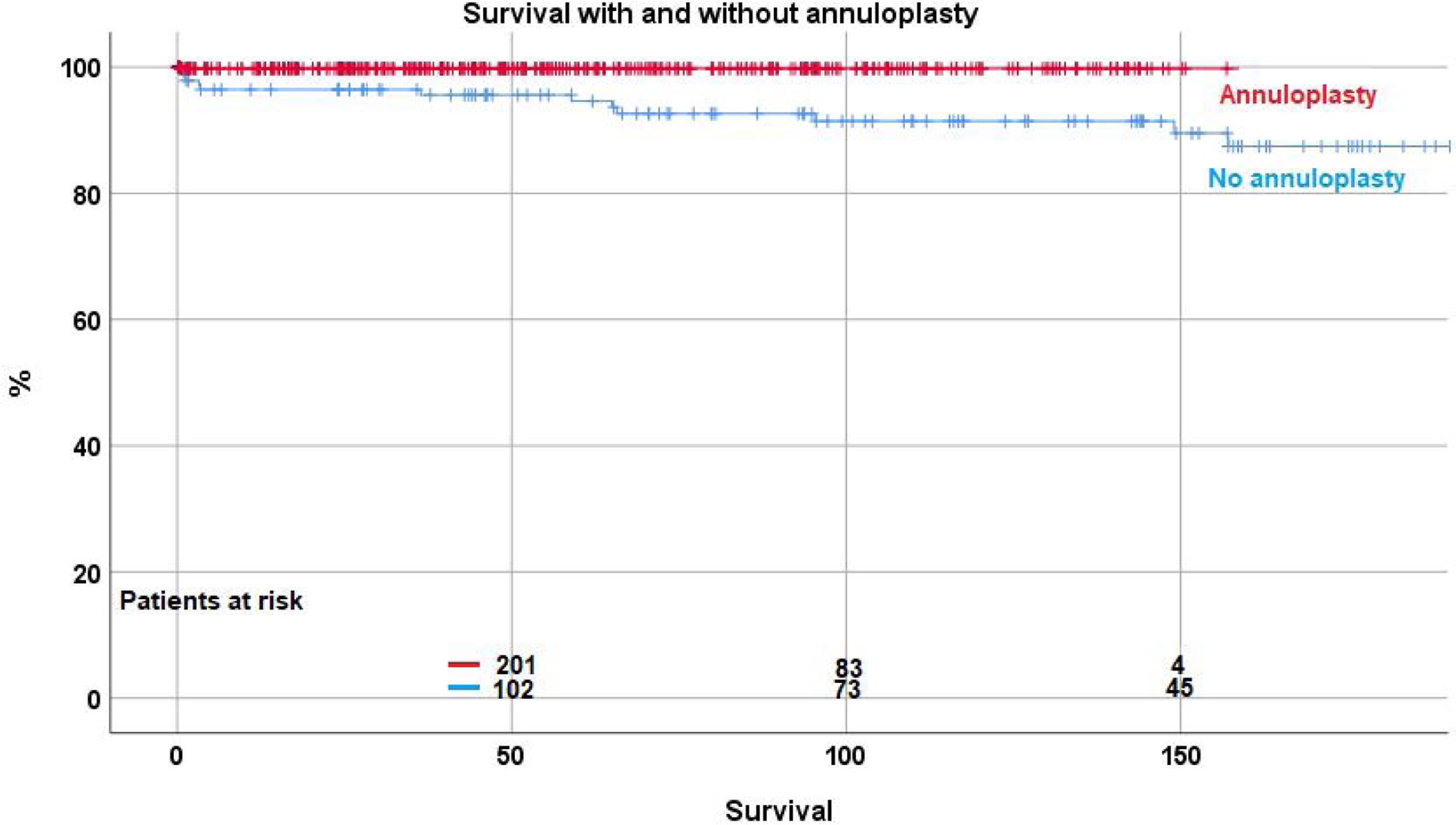
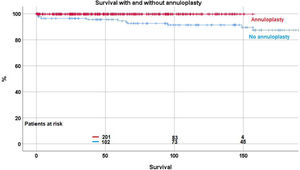
Over time, 12 patients (2.2%) died between 2 and 157 months postoperatively. Survival was 98.2%±0.7 at 5 years, 96.9%±1 at 10 years, and 93.2%±2.7 at 15 and 20 years. With the addition of suture annuloplasty, survival was 99.7%±0.3 at 10 years; it was 91.4%±2.7 without annuloplasty (p<.0001) (Fig. 1). No fatal outcome was directly or indirectly related to inadequate function of the repaired aortic valve.
By logistic regression, lack of an annuloplasty (p=0.003; OR 23) and presence of limited valve calcification (p=0.027; OR 4.1) were independent predictors of death.
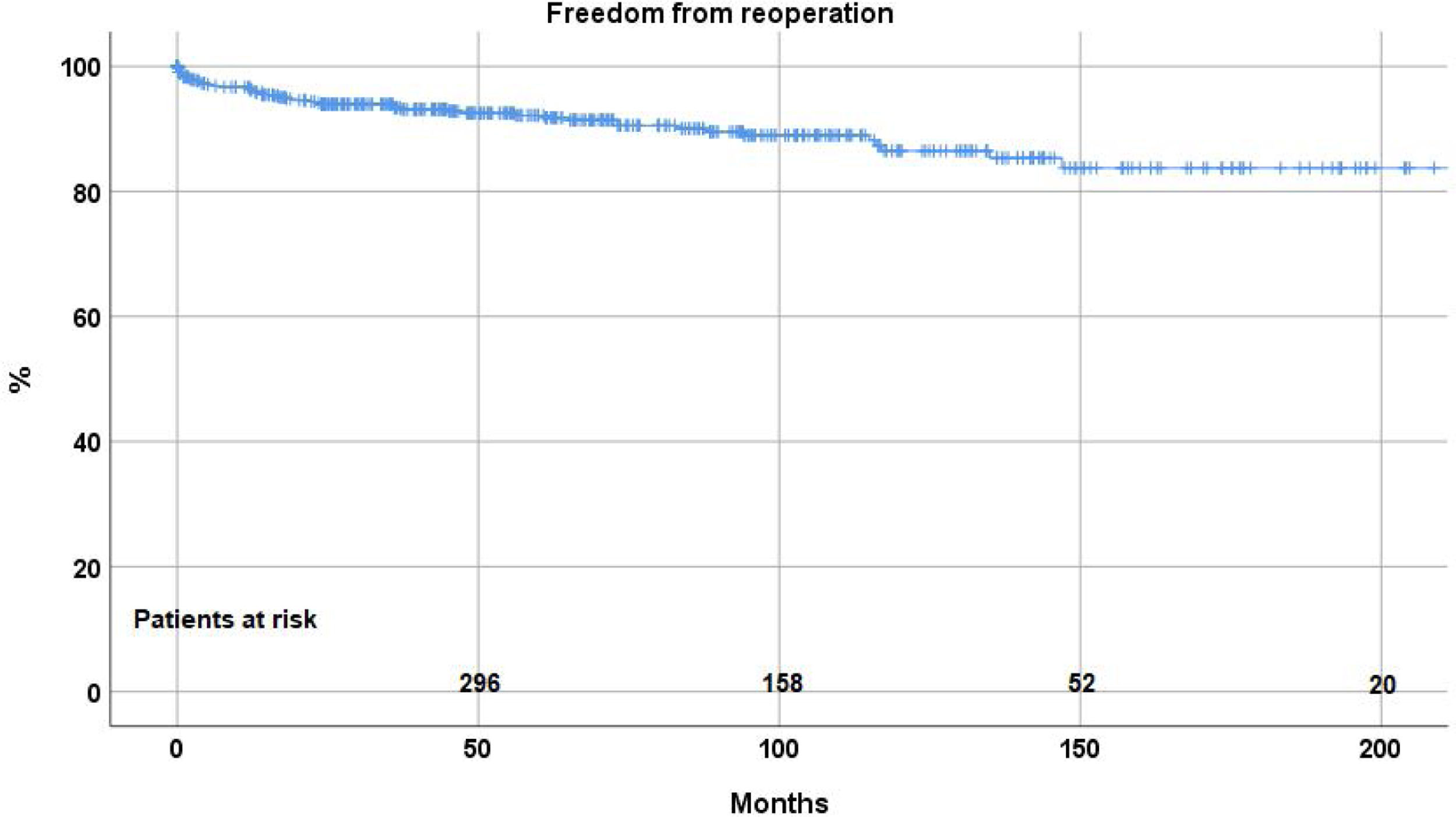
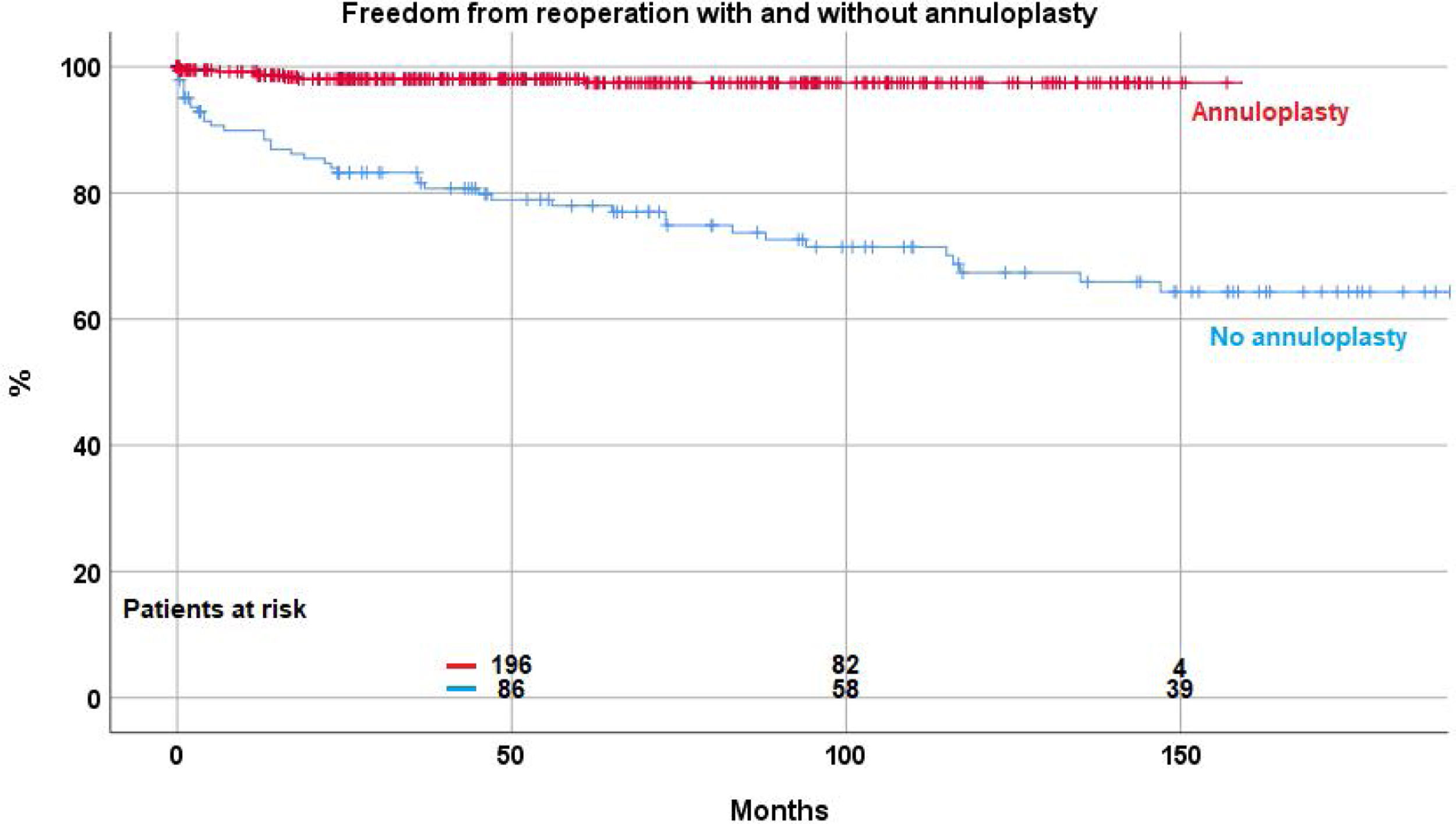
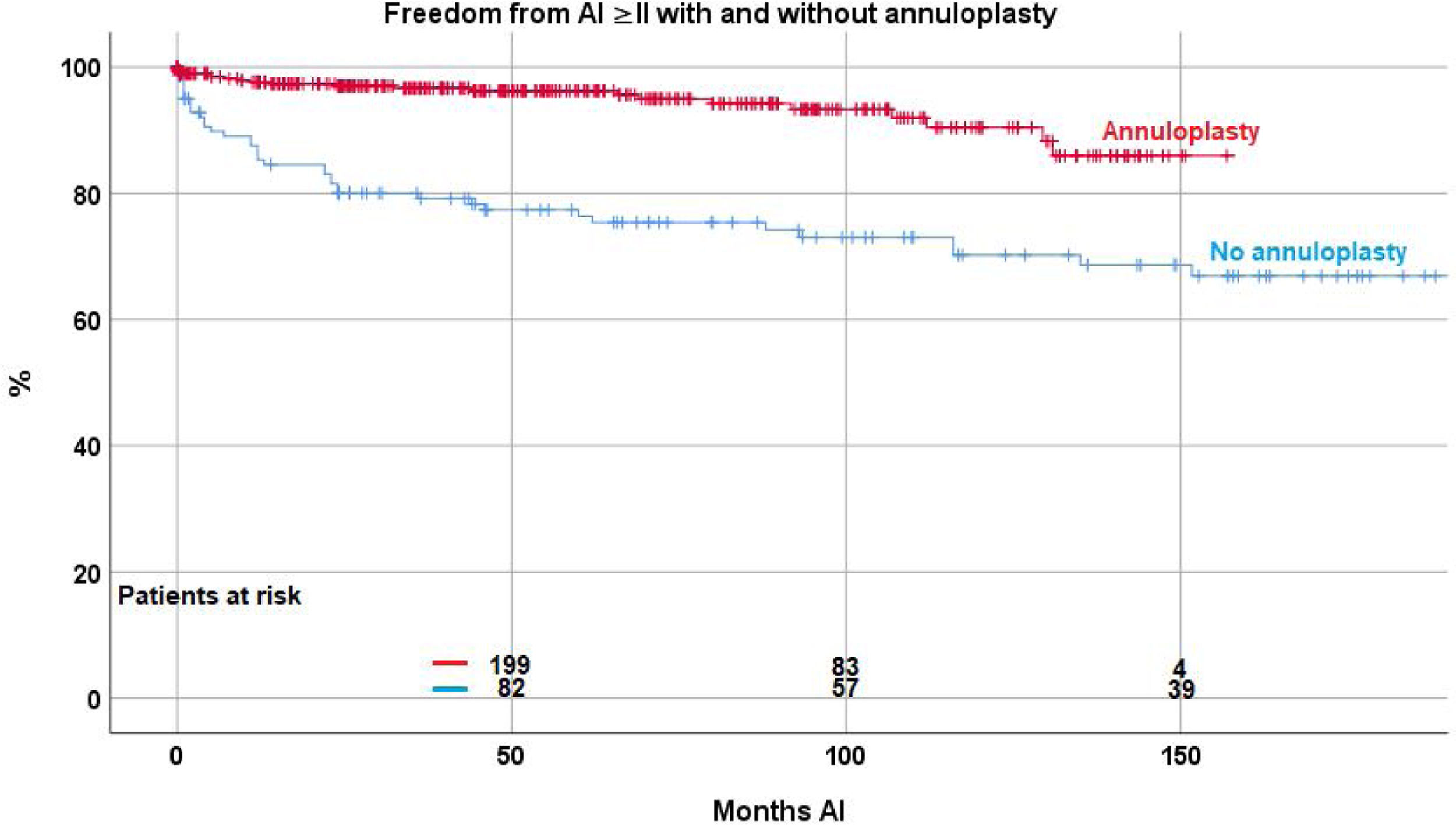
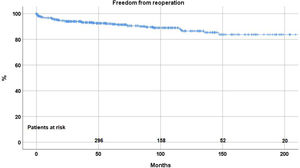
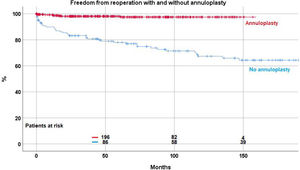
ReoperationForty-eight patient (8.8%) required a reoperation on the aortic valve between 1 and 147 months postoperatively. Freedom from reoperation was 92.2%±1.3 at 5 years, 86.5%±2.2 at 10, and 83.7±2.9 at 15 and 20 years respectively (Fig. 2). With the addition of suture annuloplasty, freedom from reoperation was 97.5%±0.9 at 10 years; it was 67.4±4.6 without annuloplasty (p<.0001) (Fig. 3).
By separate logistic regression analysis, the use of pericardial patches (p=0.019; OR 2.6), subcommissural plication (p<.0001; OR 7.3) and the lack of an annuloplasty (p<.0001; OR 17) were independent predictors for reoperation.
Freedom from AI≥IIThere was a high proportion of competent aortic valves or only trivial regurgitation at the times of discharge (AI≤I; 98.5%). The proportion of functionally competent aortic valves at discharge was higher with the use of annuloplasty (AI≤I; 99% vs 91%; p<.001).
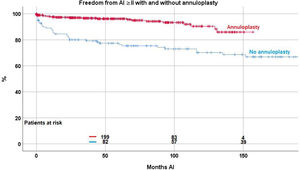
Valve function remained constant in the majority of patients, while some developed recurrent regurgitation. Freedom from AI≥II was 90.2%±1.4 at 5, 83.5%±2.3 at 10 and 77.7%±3.4 at 15 years. The Kaplan–Meier analysis showed a significant effect of suture annuloplasty in freedom from recurrent AI≥II at 10 years (with 90.4%±2.6; without 68%±4.4; p<.0001) (Fig. 4).
Independent predictors for recurrent AI≥II were the use of subcommissural plication (p≤.0001; OR 2.8), the use of pericardial patches (p≤.0001; OR 5.6), and the presence of cusp calcification (p=0.005; OR 3.9).
DiscussionWith the development of heart valve prostheses in the 1960s, aortic valve replacement became the standard treatment for aortic regurgitation. Long-term studies, however, showed valve-related complications in a relevant proportion of patients.16,17 In addition, a non-negligible rate of valve-related mortality was observed, which was associated with reduced life expectancy after aortic valve replacement.18 In addition, the risk of reoperation persisted, even after mechanical valve replacement.17 Another pitfall of mechanical valves is the lifelong need of anticoagulation, with its associated risks.16,17
By contrast, aortic valve repair has been shown to largely eliminate these risks if good repair durability was achieved. With repair, survival equivalent to that of the age- and gender-matched general population, and excellent quality of life.8
Repair of regurgitant bicuspid aortic valves has been performed for more than two decades. Initially, surgeons relied on a visual assessment of the cusps. Although early results were good, mid-term studies showed a relevant attrition of valve function. Based on the analysis of valve failures, we proposed the concept of effective height as a quantitative indicator of valve configuration.12 Others have confirmed the benefit of this concept.19 Further analysis of repair failure identified annular dilatation as an important predictor. Since the introduction of the suture annuloplasty concept, the durability of isolated bicuspid aortic valve repair could be drastically improved.14 This observation is confirmed by our current analysis. We have now been able to find a positive association between annuloplasty and not only freedom from recurrent aortic regurgitation, but also survival.
Local complications associated with suture annuloplasty were almost exclusively seen early in the experience and with the use of braided polyester sutures.14 The complications have been largely eliminated by using expanded polytetrafluorethylene instead. Interference with the circumflex artery was in only few instances and could be treated by removal of the suture. Thus, our current results of aortic valve repair show a stability that is at least comparable if not superior to that described by others applying an external annuloplasty ring.
Our initial enthusiasm about the excision of calcium and partial cusp replacement using pericardium has faded with the recognition of a high incidence of reoperations in the first years.20 Resection of limited plaques with direct approximation of cusp tissue still seems to be an acceptable approach. We have abandoned the use of pericardium for cusp repair and now propose replacement in these instances. A caveat remains with cusp calcium being a predictor of reduced survival in our current analysis. It is unclear whether this is due to preserving suboptimal valves, or whether this is merely an indicator of a patient-specific tendency to develop atherosclerosis at an earlier age.
Our current data confirm the applicability and stability of the anatomy-based repair strategy with good repair durability. Using this concept, we are now able to achieve stable repair results for the majority of patients.
LimitationsThe present study has limitations owing to its retrospective and monocentric design. It includes modifications introduced over time, nevertheless, surgical bias is limited by the fact that one surgeon substantially participated in all procedures. Moreover, our findings need to be interpreted with caution owing to multiple testing and the size of different subgroups. Despite the adjustment for possible confounders, residual confounding may have been present.
Nonetheless, to our knowledge, this is the first study on isolated bicuspid valve repair incorporating a population of more than 500 participants with a follow-up exceeding 20 years.
ConclusionIsolated bicuspid aortic valve repair is associated with excellent survival, and the majority of BAV repairs will remain stable over more than 10 years. The use of an annuloplasty improves repair durability and is associated with improved survival. Cusp calcification and cusp repair using a pericardial patch are predictors for early valve failure.
Patient and public involvementPatients were not involved in the research process of this study.
Sources of fundingNone.
Conflict of interestNone.
The authors have no acknowledgments to disclose.