The exact prevalence of nutcracker syndrome (NCS) is unknown, due to the very low incidence of this disease plus the absence of definitive diagnostic criteria. Some authors have reported a higher prevalence of this condition in women, however published and available information is limited. Due to symptoms and consequences that can cause, it is important to suspect it and diagnose it. The aim of this study was to report the outcomes of the first Latin-American experience in the treatment of NCS using an endovascular approach with a left renal vein (LRV) balloon and arterial stent angioplasty.
MethodsThe study design was a retrospective cohort analysis of 11 patients who underwent endovascular approach with LRV balloon and arterial stent angioplasty for the treatment of NCS between January 2019 and April 2022 in Bogota, Colombia.
ResultsDuring January 2019 to April 2022, 553 patients were treated for congestive pelvic syndrome. Only 11 patients have clinical criteria (hematuria, varicose pelvic veins, and left flank pain), imaging diagnostic criteria (abdominal veins Doppler ultrasound or abdominal computed tomography with superior mesentery artery – abdominal aorta angle <39°) and an average of LRV – inferior vena cava pressure measurement comparison difference of 6mmHg for NCS. The 100% were female patients. All the patients were under 65 years and underwent endovascular approach (EVA) with LRV balloon and arterial stent angioplasty. The 100% of patients presented resolution of the collateral vein circulation in the final control phlebo-cavography. The average age was 46.5 years old. Ten patients had an anterior type of NCS and only 1 had a posterior type of NCS. None of the patients had NCS due to secondary causes such as pancreatic head tumors.
ConclusionsThis study shows that the endovascular approach with LRV balloon and arterial stent angioplasty is a safe and cost-effective treatment for NCS with low incidence of complications and no need of postoperative reintervention in short and middle term follow-up.
Debido a la muy baja incidencia de esta enfermedad más la ausencia de criterios diagnósticos definitivos, se desconoce la prevalencia exacta del síndrome de nutcracker o cascanueces (SNC). Algunos autores han reportado una mayor prevalencia de esta condición en mujeres, sin embargo la información publicada y disponible es limitada. Debido a los síntomas y consecuencias que puede ocasionar, es importante sospecharlo y diagnosticarlo. El objetivo de este estudio fue reportar los resultados de la primera experiencia latinoamericana en el tratamiento del SNC mediante un abordaje endovascular con balón y angioplastia con stent arterial por la vena renal izquierda (VRI).
MétodosEl diseño del estudio fue un análisis de cohorte retrospectivo de 11 pacientes que se sometieron a abordaje endovascular con balón VRI y angioplastia con stent arterial para el tratamiento de SNC entre enero de 2019 y abril de 2022 en Bogotá, Colombia.
ResultadosDurante el periodo de enero de 2019 a abril de 2022, 553 pacientes fueron tratados por síndrome pélvico congestivo. Solo 11 pacientes tienen criterios diagnósticos clínicos (hematuria, várices pélvicas y dolor en el flanco izquierdo), imagenológicos (Doppler de venas abdominales o tomografía computarizada abdominal con ángulo de arteria mesentérica superior-aorta abdominal <39°) y un promedio de diferencia de presión VRI-vena cava inferior de 6mmHg para SNC; 100% fueron pacientes del sexo femenino. Todos los pacientes menores de 65 años fueron sometidos a abordaje endovascular (AEV) por VRI con balón y angioplastia con stent arterial; 100% de los pacientes presentó resolución de la circulación venosa colateral en la flebocavografía final. La edad promedio fue de 46,5 años. Diez de los pacientes tenían un tipo anterior de SNC y solo uno tenía un tipo posterior de SNC. Ninguno de los pacientes presentaba SNC por causas secundarias como tumores de cabeza de páncreas.
ConclusionesEste estudio demuestra que el abordaje endovascular por VRI con balón y angioplastia con stent arterial es un tratamiento seguro y rentable para el SNC con baja incidencia de complicaciones y sin necesidad de reintervención postoperatoria a corto y mediano plazo.
.
Nutcracker syndrome (NCS) or left renal vein (LRV) entrapment syndrome, was first described by Grant in 1937. There are two main forms of NCS, anterior and posterior. The most frequent type of NCS is the anterior type, which is defined as the compression of the LRV between the aorta and superior mesentery artery (SMA) with an angle between the SMA and the aorta less than <39° in the computed tomography (CT). This last one imaging criteria in the CT had a sensitivity of 92% and specificity of 89% for detecting symptomatic NCS. However, these results are limited due to the small sample size of cases reported in the evidence, with a maximum sample of 61 patients with NCS published in the actual literature. Posterior type is the less common type of NCS with only 19 cases reported in the literature, that developed when the retro-aortic or circum-aortic renal vein is compressed between the aorta and the vertebral bodies.
The classic definition of NCS is when the left renal vein is compressed between the aorta and the superior mesenteric artery (SMA), as a nut between the jaws of a nutcracker. This condition leads to stenosis of the LRV at the aorto-mesenteric anatomic clamp, with dilatation of the distal portion of the vessel. In 1972 a Belgian radiologist, Dr De-Schepper, gave the name of NCS to this entity.
The NCS generates a classic triad of clinical signs and symptoms, conformed by hematuria, left flank pain and varicose pelvic veins in females, and varicocele in male patients. This clinical association is important, because asymptomatic LRV dilatation found on Doppler ultrasound (DUS) and computed tomography (CT) must be considered a normal finding.1,2
The exact prevalence of NCS is unknown, due to the very low incidence of this disease plus the absence of definitive diagnostic criteria. Some authors had reported a higher prevalence of this condition in females. The aim of this study was to report the outcomes of the first Latin-American experience in the treatment of NCS using an endovascular approach with LRV balloon and arterial stent angioplasty.
Materials and methodsStudy populationThe study design was a retrospective analysis of a single cohort of 11 patients (from a global population of 553) who underwent endovascular approach by the LRV with balloon and angioplasty with arterial stent for the treatment of NCS between January 2019 and April 2022 in Bogota, Colombia. The study protocol was approved by the ethics committee. The protocol was implemented in accordance with ethical guidelines of the “World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and revised in Tokyo 2004.
Data collectionDuring study period, 553 patients were treated for congestive pelvic syndrome and varicose pelvic veins, of which 11 underwent an endovascular approach with LRV balloon and arterial stent angioplasty for NCS in the Department of Vascular Surgery of Clinica Colsubsidio Calle 100 Bogotá – Colombia. All the patients were treated by vascular and endovascular surgeons.
Data relating to each patient were entered retrospectively, standardized, and analyzed using Microsoft Excel 2017. Including variables such as age, sex, symptoms, type of NCS, SMA – abdominal aorta angle, diagnostic image, procedure, operative time, bleeding, hospital length of stay, intensive care unit (ICU) length of stay, symptoms improvement, hematuria improvement, complications, reintervention, gonadal iliac anastomosis thrombosis and mortality. All patients who had a NCS with an abdominal CT that showed an SMA – abdominal aorta angle less than 39° and the presence of symptoms treated.
Postoperative complications were classified according to the Clavien–Dindo3 grading system. Infectious complications were defined as a clinical or culture positive for nosocomial infections in accordance with the Society of Critical Care Medicine guidelines. Postoperative complications recorded as Clavien–Dindo grade III or greater were regarded as severe. Mortality was defined as any cause of death occurring in hospital after the diagnosis and surgical treatment of NCS in the next 30 days as an early mortality and in the next years as a late mortality. All patients in this study underwent an endovascular approach with LRV balloon and arterial stent angioplasty for NCS. Operative management of the NCS was according to a specific operative strategy based on the age and comorbidities of the patient. Postoperative patients follow-up for 12 months in an outpatient setting.
Preoperative assessment – inclusion criteriaAll the patients that had congestive pelvic syndrome confirmed with abdominal veins Doppler ultrasound or angioCT, were taken to diagnostic and/or therapeutic cavography and to pelvic veins phlebography. During the cavography and pelvic veins phlebography the patients could present centripetal vein flow, varicose pelvic veins, and signs of NCS with lumbar veins flow derivation, severe incompetency, and dilatation of left gonadal vein, circumaortic veins, stenosis of the LRV-caval union and fumarole LRV sign. If the patient presented any of the previously mentioned signs, then was taken to LRV – inferior vena cava (IVC) pressure measurement comparison using a multi-purpose terminal unique orifice catheter with an arterial line transductor in the venous central pressure modality (the LRV–IVC pressure measurement comparison was positive for NCS if the difference of pressure was equal or higher to 3mmHg).
If the patients presented the criteria mentioned above (clinical, imaging, angiographic and pressure differences, also signs and symptoms) were selected for an endovascular approach (11/553 patients).
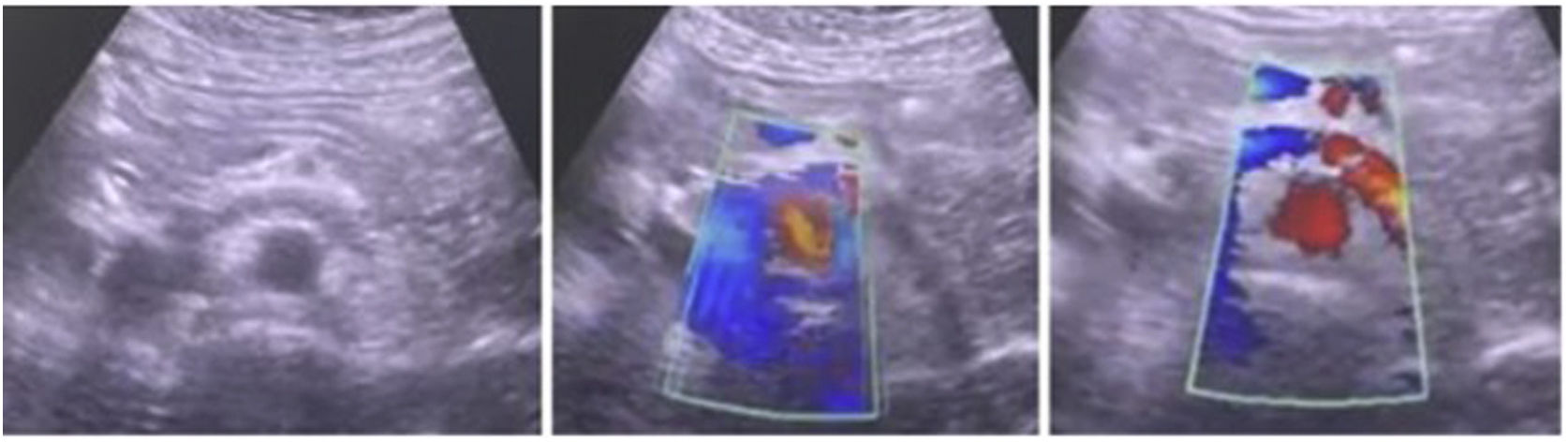
Surgical techniqueThe principles applied were urgent LRV pressure control, treatment of the hematuria, venous drainage derivation and congestive pelvic syndrome origin resolution. All the patients remain 24h postoperative in hospital observation. The repair was performed via endovascular access, after local anesthesia and sedation. The right common femoral and the left internal jugular vein was punctured with ultrasound guide and a 6F introducers sheath were inserted. The LRV was catheterized with a Cobra 2 5F diagnostic catheter and phlebography was performed. This confirmed critical stenosis of the LRV and the pelvic varicose veins on the left, with a dilated left gonadal vein (Fig. 1). The stenosis pressure gradient was measured in all patients. Through the internal jugular access, balloon LRV angioplasty was performed (Fig. 2) and then deployment of a e-Luminex self-expanding nitinol bare metal arterial stent (10–12mm×40mm) (Figs. 3 and 4) was performed resolving the NCS (Fig. 5). Additionally, identified pelvic varicose veins were embolized using Concerto coils (20mm×50mm) through the basilic vein using a multi-purpose catheter. No dietary restrictions were imposed, and oral food intake was initiated in the first 3 postoperative hours. No need for a postoperative ICU. Postoperative anticoagulation for 6 months using rivaroxaban and then switching to salicylic acid for another 6 months was prescribed to all patients. The postoperative patients follow-up was performed using Doppler ultrasound in the 6 months and physical examination in all face-to-face clinical outpatient consults.
During the period from January 2019 to April 2022, 553 patients were treated for congestive pelvic syndrome. Only 11 patients have clinical (hematuria, varicose pelvic veins, and left flank pain), imaging diagnostic criteria (abdominal veins Doppler ultrasound or abdominal CT with a SMA – abdominal aorta angle less than 39°) and an average of LRV–IVC pressure measurement comparison difference of 6mmHg for NCS. The 100% were female patients. All the patients were under 65 years old and underwent endovascular approach with LRV balloon and arterial stent angioplasty. The 100% of patients had collateral venous flow restored in the final control phlebo-cavography. The average age was 46.5 years old. Ten of the patients had an anterior type of NCS and only 1 had a posterior type of NCS (Fig. 6). None of the patients had NCS due to secondary causes such as pancreatic head tumors.
The mean operative time was 54min. The average bleeding time was 5cc. Length of hospital stay was 1 day (minimal bleeding and length of hospital stay). None of the patients required UCI. No complications or interventions were observed. The mortality was 0%. All patients were taken to pelvic varicose vein endovascular embolization. The 11 patients presented an average symptom resolution of 100%. No stent or LRV thrombosis was reported. No stent migration or other related complications were presented. No need of open or endovascular reintervention was presented in this case series. We suggest follow-up with iliocaval echo-Doppler every 6 months to assess the integrity of the venous system.
DiscussionOther conditions can emulate NCS, such as abdominal aortic aneurysm, neoplasms as pancreatic or retroperitoneal, also para-aortic lymphadenopathy, etc.1 In NCS the pressure gradient between the LRV and the IVC can rise from a normal level of 1mmHg to 3mmHg or more. This produces a rupture of the thin-walled septum between the small veins and the collecting system of the renal fornix generates hematuria.1,2,4 The principal open vascular approaches to treat NCS include transposition of the LRV and renal autotransplant. The transposition of the LRV is considered a gold standard in the treatment of NCS until now. LRV transposition consists in a section of the LRV at the IVC junction with reimplantation of the LRV distal to the SMA. This surgical open procedure is performed through a midline transperitoneal approach with the risks of postoperative ileus, bleeding, and LRV thrombosis. The LRV transposition appears to be a safe and effective procedure, although it has been reported that selected patients can have persistent symptoms with a higher rate of intraoperative bleeding and length of hospital stay. Renal autotransplant is a more invasive procedure with left nephrectomy and left kidney autotransplantation in either the left or right iliac fossa. However, renal autotransplantation carries additional risks including increased renal ischemia time, the need of a wider surgical dissection, and two additional vascular and urologic anastomoses (renal artery and ureter).5 Using the endovascular approach with LRV balloon and arterial stent angioplasty for the treatment of NCS had excellent reported surgical outcomes, with a 100% of left flank pain and hematuria resolution in the long term, like in our study with minimal bleeding and length of hospital stay.
An alternative approach to the treatment of NCS is the SMA-aortic transposition. This approach has a limited exposure of the retroperitoneum and reduces the risk of LRV thrombosis due to the no need of surgical manipulation. However, this procedure has a significant potential risk of mesenteric ischemia that explain why only a limited number of cases have been reported in the scientific literature. Other miscellaneous surgical interventions for NCS include proximal testicular-iliac-saphenous anastomosis (involving the use of the saphenous vein as a conduit to link the testicular and iliac veins), gonado-caval bypass, peri-pelvic varicosity excision, renocaval venous bypass, renal decapsulation and transluminal balloon angioplasty.5-7
Hohenfellner et al., in their case series, reported that LRV transposition could relieve symptoms in 7 of their eight patients with follow-up ranging from 41 to 136 months; they reported postoperative complications including deep vein thrombosis (DVT), retroperitoneal hematoma, paralytic ileus and adhesive small bowel obstruction (ASBO). However, in the endovascular approach with LRV balloon and arterial stent angioplasty, we avoid the risk of incisional postoperative hernia, small bowel ileus, postoperative adhesions and ASBO in the short and long term with less postoperative pain and a faster return to daily work.1,8
In a series reported by Gloviczki et al. with 11 patients who underwent LRV transposition, 3 required reintervention and 2 had LRV thrombosis. In our cohort none of the patients required endovascular or open surgical reintervention. In the mean follow-up of 12 months our 11 patients presented relief of symptoms like left flank pain and hematuria in 100%. Hartung et al. reported a case series of 42 patients treated with different open surgical approaches with excellent results in 35 patients. In patients treated with LRV transposition, 17 of 18 became asymptomatic with recurrent hematuria in 1 patient. Recurrent hematuria has been associated with longstanding venous hypertension and subsequent hyperplastic mature venocalyceal communication that could lead to the need of nephrectomy if the hematuria could not be controlled. In our series of endovascular approach with LRV balloon and arterial stent angioplasty, we do not present recurrence of hematuria or needed of nephrectomy.1,6
The laparoscopic approach in NCS is limited to case reports. However, the outcomes of laparoscopic procedures reported in the literature are comparable with open approaches; these minimally invasive approaches include laparoscopic spleno-renal venous bypass, laparoscopic LRV transposition and laparoscopic inferior mesenteric-gonadal bypass. However, laparoscopic spleno-renal venous bypass involves draining the LRV into the portal system with the risk of persistence of LRV hypertension, especially when collaterals of the LRV are ligated. Chung et al. found that after laparoscopic spleno-renal venous bypass the luminal diameter of the splenic vein increased to twice of its normal diameter once unclamped. Laparoscopic inferior mesenteric-gonadal bypass avoids the risk of renal reperfusion injury and LRV hypertension plus no need of anastomosis to the splenic vein, reducing the risk of splenectomy and the anatomically restricted access due to the location of the pancreas. In terms of outcomes, at 8 months follow-up the patient's chronic left flank pain improved with no need for analgesia. In our study using the endovascular approach with LRV balloon and arterial stent angioplasty, we avoid the risks of LRV hypertension, renal ischemia and small bowel ileus with a lower operative time and less intraoperative bleeding.1,9
The endovascular approach (EVA) avoids the requirements for additional anastomosis, extensive surgical dissection, and long periods of renal ischemia presented in open surgery. Disadvantages of EVA are the need of postoperative anticoagulation plus the elevated stent cost. A retrospective analysis of the largest case series of EVA showed that most patients (96.7%, 59 of 61) treated by EVA experienced some degree of symptomatic improvement including flank pain and hematuria at 6-month follow-up and no significant cases of restenosis at 66-month median follow-up. Nevertheless, the EVA had reported complications including incorrect stent placement requiring surgical intervention, stent migration, partial stent dislodgement into the IVC and stent migration into the hilar region of the LRV. These reported complications highlight the importance of adequate stent sizing. Less common complications of the EVA include stent embolization, in-stent restenosis, pseudo-intimal proliferation or thrombosis and stent fracture with vessel occlusion. The EVA must be the ideal approach in centers with all the resources and experience in open and endovascular approaches like ours. In our center using the endovascular approach with LRV balloon and arterial stent angioplasty have similar outcomes to the reported evidence by Chen et al. with no stent related complications. The use of an arterial bare metal stent for the LRV angioplasty allows us to use a lower profile vascular access in the internal jugular vein (6 Fr) with a better navigability and conformability to the curve and angle of the LRV and the IVC. This arterial stent had the most ideal diameters and lengths to adapt in the LRV that is 10–12mm and 40mm. The other plus to use an arterial stent is the higher radial force to avoid stent migration or dislodgement.1,7
If compared with other techniques such as the LRV–IVC transposition, our technique is cost-effective since the stent averages a cost of 350 dollars, the balloon 150 dollars, and the procedure in general 700 dollars on average, with only one day of hospitalization without ICU requirement. On the side of transposition or other procedures, it averages 1000 dollars plus 3–5 days in ICU. With higher risk of complications and new interventions.
Study limitationsThe findings of this study should be interpreted within the context of its design. It is a single center nonrandomized retrospective non comparative study with a small sample. The results should therefore be viewed as hypothesis-generating to conduct future studies. All data were retrospectively collected from the electronic medical records and the outcomes are based on what has been registered. Strengths of this study are the detailed short and long-term clinical outcomes of the endovascular approach with LRV balloon and arterial stent angioplasty for the treatment of NCS.
ConclusionThis study shows that the endovascular approach with LRV balloon and a vein angioplasty with arterial stent is a safe and cost-effective treatment for NCS with low incidence of complications and no need of postoperative reintervention in short and middle term follow-up. This is the first step for the conduct of comparative studies with the open treatment for NCS.
Nutcracker syndrome occurs when the left renal vein is compressed between the aorta and the superior mesenteric artery, it is more common in women. However, there is little literature to assess the specific epidemiological and clinical information of the NCS. In addition, there are no defined criteria to achieve a timely and accurate diagnoses. There are no enough reports or data that allow evaluating the frequency and management of the syndrome in Latin-American population.
What does this study add?Endovascular approach with LRV balloon and arterial stent angioplasty for the treatment of nutcracker syndrome, is a safe and cost-effective treatment for NCS with low incidence of complications and no need of postoperative reintervention. We show, in 11 patients in Bogota, Colombia that the 100% of patients present symptom resolution and resolution of the collateral vein flow circulation in the final control phlebo-cavography.
The institution does not have an ethics committee but its general management authorized the study, since it was a retrospective study without risk or interventions direct to patients, derived from it. On the other hand, researchers and institution protected the data and identities of the participants.
Conflict of interestsThe authors declare they have no conflict of interest.