Transesophageal echocardiography is crucial to evaluate patients with mitral regurgitation undergoing mitral valve surgery. Characterization of the anatomy of the valve, mechanism and severity of the regurgitation are the first steps to plan the type of intervention. Two-dimensional echocardiography has been widely available and a prerequisite before referring the patient to mitral valve surgery. However, the advent of 3-dimensional echocardiography has allowed visualization of the mitral valve resembling the view the surgeons have at the operating theater and has provided more accurate quantification of the severity of the mitral regurgitation. Accordingly, 3-dimensional echocardiography has become a must in the evaluation of patients with severe mitral regurgitation. In this review article, the role of 2- and 3-dimensional echocardiography in the evaluation of mitral regurgitation and the key aspects needed before mitral valve surgery are described.
La ecocardiografía transesofágica es fundamental para evaluar a los pacientes con insuficiencia mitral sometidos a cirugía de la válvula mitral. La caracterización de la anatomía de la válvula, el mecanismo y la gravedad de la regurgitación son los primeros pasos para planificar el tipo de intervención. La ecocardiografía bidimensional ha estado ampliamente disponible y es un requisito previo antes de derivar al paciente a cirugía de la válvula mitral; sin embargo, el desarrollo de la ecocardiografía tridimensional ha permitido una visualización de la válvula mitral similar a la visualizacion de los cirujanos en el quirófano y ha proporcionado una cuantificación más precisa de la gravedad de la insuficiencia mitral. En consecuencia, la ecocardiografía tridimensional es esencial en la evaluación de pacientes con insuficiencia mitral grave. En este artículo de revisión se describe el papel de la ecocardiografía bidimensional y tridimensional en la evaluación de la insuficiencia mitral y los aspectos que deben ser evaluados antes de la cirugía de la válvula mitral.
Mitral regurgitation (MR) is the second most frequent valvular heart disease.1 The incidence of MR increases with age and the number of patients with MR requiring mitral valve intervention is expected to rise sharply in the next decades.2,3
Left untreated, severe MR is associated with poor prognosis due to the detrimental consequences of the chronic volume overload on the left ventricle (LV). Mitral valve repair results in improved outcomes of patients with primary MR4,5 whereas mitral valve repair or replacement is indicated in patients with secondary MR who require coronary artery bypass grafting. Recent data from a single randomized clinical trial have shown that transcatheter edge-to-edge repair leads to better prognosis of selected patients with secondary MR and contraindications for surgery.6
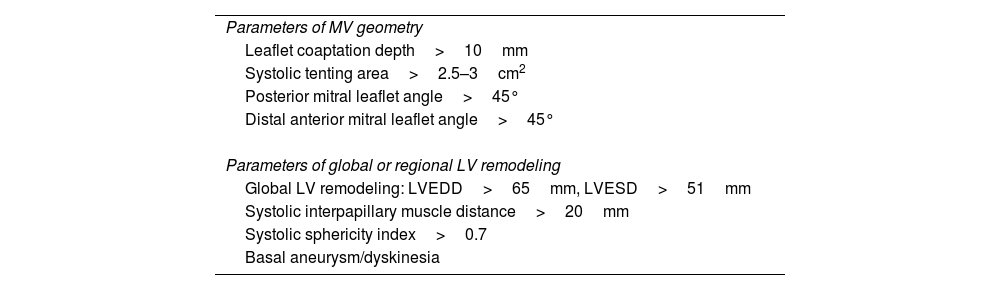
Accurate diagnosis of the severity and mechanism of MR is key for decision making and appropriate timing of intervention in patients with severe MR. Transthoracic echocardiography (TTE) is the first-line imaging technique for the assessment of MR. Nevertheless, when further diagnostic refinement is needed or surgical or transcatheter valve procedures are considered, transesophageal echocardiography (TEE) plays an essential role.7 Detailed TEE is particularly well suited to characterize the mechanism and severity of MR and provides essential morphological data to evaluate the reparability.7,8 The diagnostic accuracy of TEE may be further increased using 3-dimensional (3D) imaging, which improves the visualization of MV anatomy and morphology, allowing a better assessment of valve lesion(s) and their eligibility for surgical repair.7,8 Novel technologies to visualize the mitral valve with 3D echocardiography allow characterization of the mitral valve tissue and permit good differentiation between calcium deposition (mainly along the mitral valve annulus) and the leaflets as well as an estimation of the thickness of the leaflet tissue (Fig. 1). These tools are being currently evaluated to establish their impact on the surgical technique of repair.
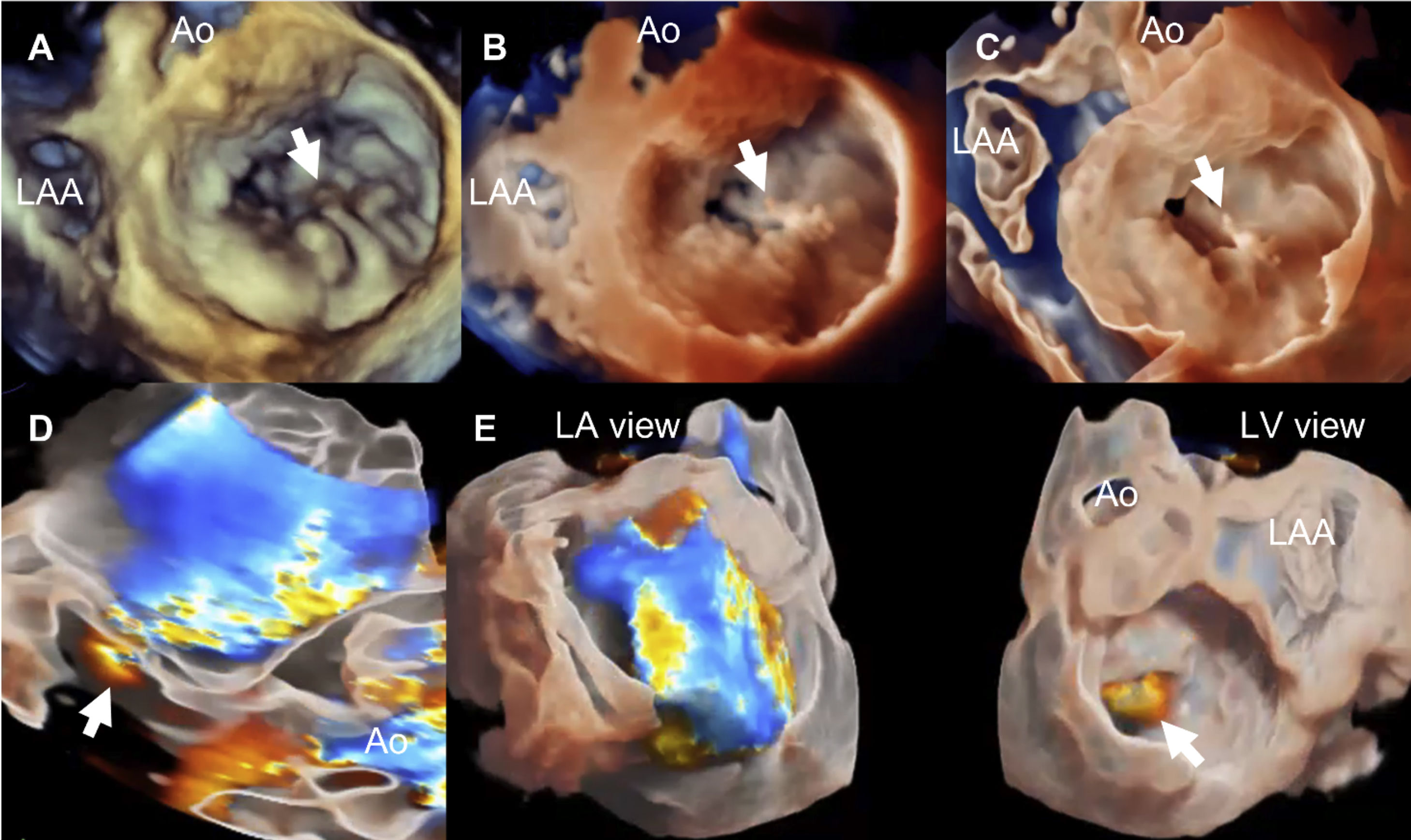
Visualization modes of 3-dimensional full volume of the mitral valve. Legend. Panel A shows the conventional visualization of the 3-dimensional full volume of the mitral valve, resembling the orientation of the surgical inspection, with the aortic valve (Ao) at 12 o’clock and the left atrial appendage (LAA) in the vicinity of the anterior commissure of the mitral valve. In this example, the arrow indicates a redundant, prolapsing central scallop of the posterior mitral leaflet. Photo realistic 3-dimensional rendering of the same image (TrueVue © Philips) is shown in panel B where the chorda rupture can be better visualized (arrow). Panel C shows the cardiac TrueVue Glass © (Philips) which permits another visualization of the mitral valve by peeling away layers and leaving anatomical landmarks. The arrow indicates the chorda rupture and the prolapse of the central scallop of the posterior mitral leaflet. In these two modes of visualization, the observer can place the light to create shadows and enhance the visualization of the structure of interest. In panel D, the 3-dimensional color Doppler flow of the mitral regurgitant jet is shown and visualized with TrueVue Glass to better delineate the origin of the regurgitant jet (arrow). Furthermore, the left atrial and left ventricular side of the mitral valve can be simultaneously visualized helping to understand the origin of the regurgitant jet (arrow) between the central and medial segments of the mitral valve (panel E).
Transesophageal echocardiography (TEE) is the ideal imaging modality for the assessment of the MV, due to the close position between the probe and the valvular apparatus, without interposition of lung or chest wall.7 Etiology and mechanism of MR, severity of MR and eligibility for MV repair are the main three questions that need to be answered during the TEE examination.7,8
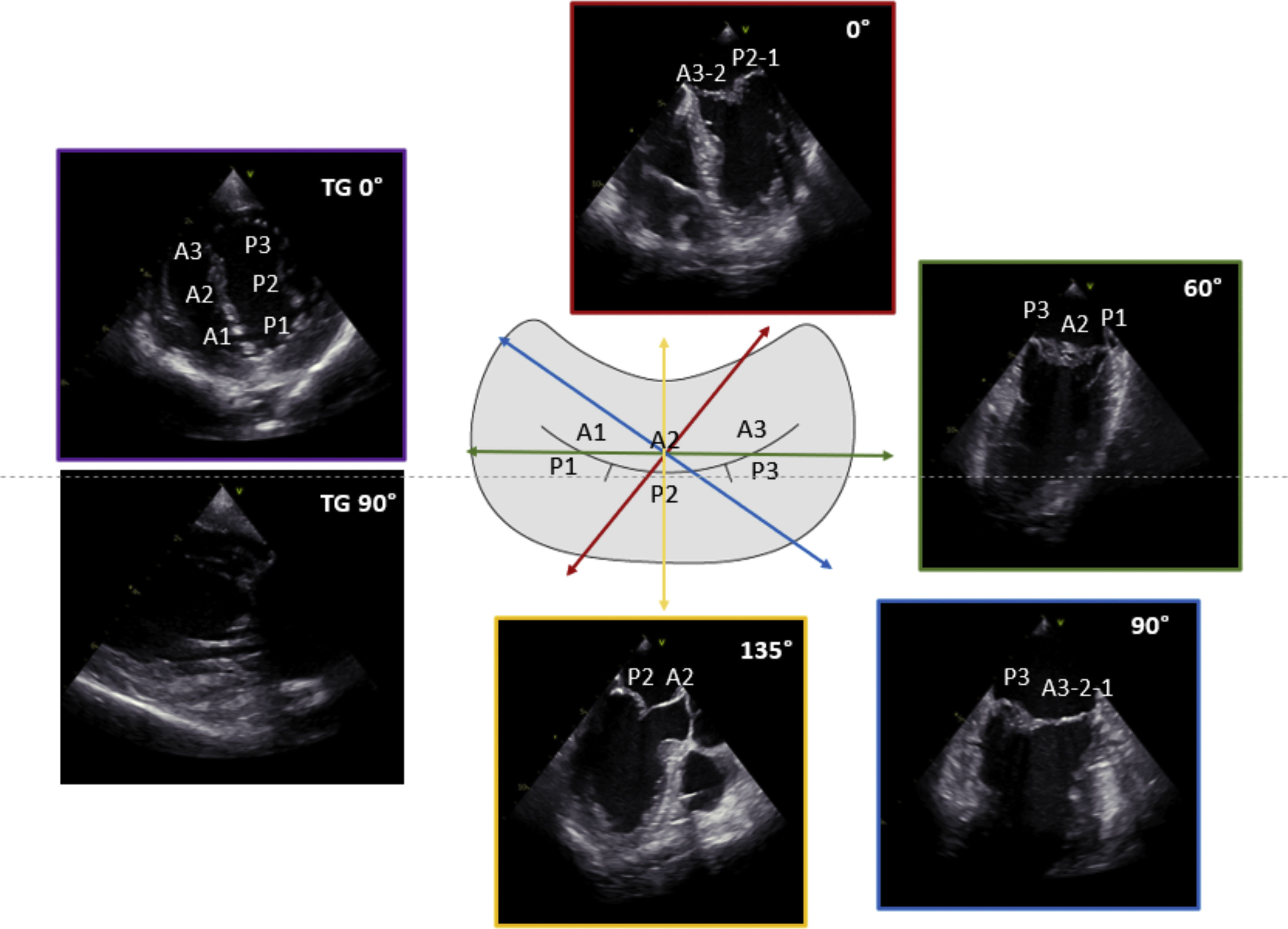
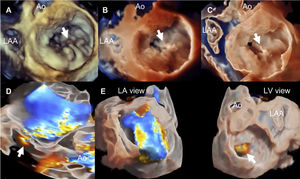
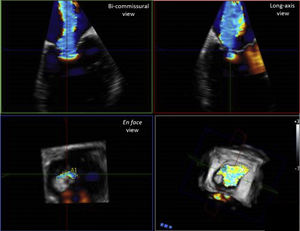
The key TEE planes include mid-esophageal (ME) views at 0° (ME four-chamber view), 60° (bi-commissural view), 90° (ME two-chamber view) and 135° (ME long-axis view) and trans-gastric (TG) views at 0° (TG basal short-axis view) and 90° (TG two-chamber view) (Fig. 2). Of note, the above-mentioned probe angles are only a guidance, while these views should be optimized according to the reference structures. Anatomical landmarks are crucial in identifying the components of MV apparatus,9 such as the aortic valve adjacent to anterior leaflet, the left atrial appendage identifying the lateral portion of MV (scallops A1-P1) and the inter-atrial septum identifying the medial portion of MV (scallops A3-P3). Each of these views should be visualized with and without color Doppler and, when appropriate, pulsed-wave and continuous-wave Doppler data should be acquired to assess the severity of MR and rule out the presence of mitral stenosis.
3D imaging has become an important component of the TEE assessment in patients with MR, due to the superior image quality to assess additional morphological and functional information.10 The 3D study of the MV is typically performed from the ME views, with the use of different imaging modes, reported below8,11:
- 1)
Simultaneous multiplane imaging enables the simultaneous 2D visualization of the MV scallops and the MR jets from multiple perspective.
- 2)
Live imaging enables an initial rapid 3D assessment of the MV apparatus and is the modality of choice when the multi-beat reconstruction is technically difficult (i.e. variables R-R intervals because of atrial fibrillation or respiratory motion).
- 3)
R-wave gated multi-beat acquisition offers a detailed 3D assessment of the MV with high temporal and spatial resolution, but with the potential disadvantage of stitching artifacts in case of irregular heart rhythms or respiratory movements.
According to the size of the structure of interest (i.e. scallop lesion or the entire MV apparatus), 3D live or multi-beat imaging can be acquired using a narrow-sector (30°×60° pyramidal volume), focused wide-sector (zoom) or full-volume mode. In addition, each of these imaging modes can be integrated with color Doppler flow information.
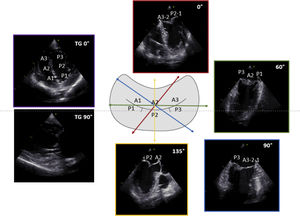
3D imaging enables the visualization of the valvular apparatus from any angle or in any plane. Particularly, the manipulation of the 3D dataset allows the MV to be displayed en face in an orientation identical to the surgeon's view intraoperatively (from the left atrial perspective).11 In this view, by displaying the aortic valve at the top center of the image (12 o’clock) and the left atrial appendage to the left (9 o’clock), the scallops of each leaflet – first to third – are visualized from left to right, respectively (Fig. 3A). This surgical view facilitates the communication with the cardiac surgeon and, importantly, improves the visualization of segmental MV anatomy, reducing the risk of scallop misinterpretation in comparison to 2D TEE.10
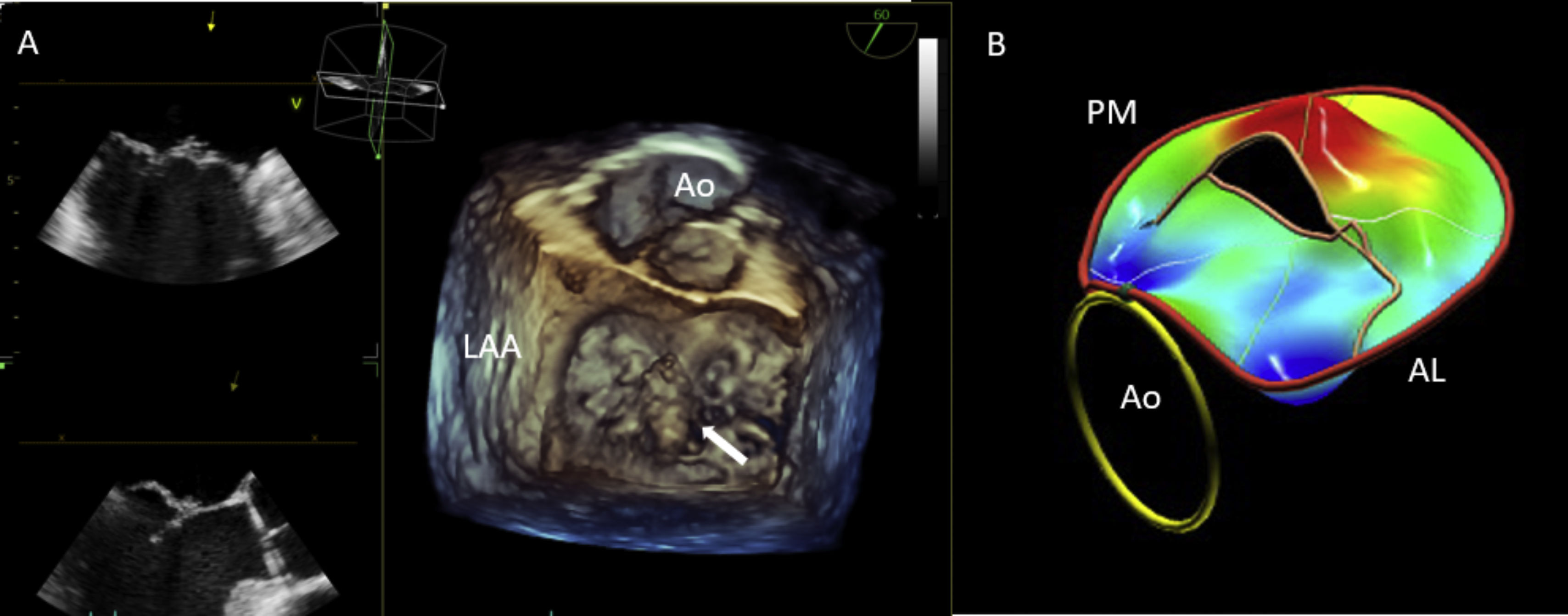
3D TEE imaging with mitral valve parametric mapping. Legend. Panel A illustrates the acquisition of 3D TEE imaging using a zoom mode and the MV visualization from the surgical view in a patient with fibroelastic deficiency. The ruptured chord resulting in P2 flail can be clearly identified (white arrow). Panel B displays the systolic parametric map, obtained using commercial software for MV quantification (4D Auto MVQ, GE Vingmed Horten, Norway). The color on the map indicates the degree of leaflet displacement into the left atrium from the mitral annulus. In this example, the deep-red hue shows the P2 flail with a large coaptation gap. Abbreviations. 3D: three dimensional; Ao: aorta; LAA: left atrial appendage; AL: antero-lateral; PM: postero-medial.
MR may be broadly categorize as either primary, when is caused by an intrinsic pathology of mitral leaflet and/or sub-valvular apparatus or secondary (functional), when MV anatomy is normal but abnormalities of the LV and/or LA compromise normal valvular function. The distinction between primary versus secondary MR is crucial for the therapeutic approach.12 Additionally, MR needs to be further characterized describing the (1) etiology, (2) the resulting lesion(s) and (3) the mechanism of valve dysfunction,13 and the precise definition of these elements is essential for determining the eligibility for MV repair.
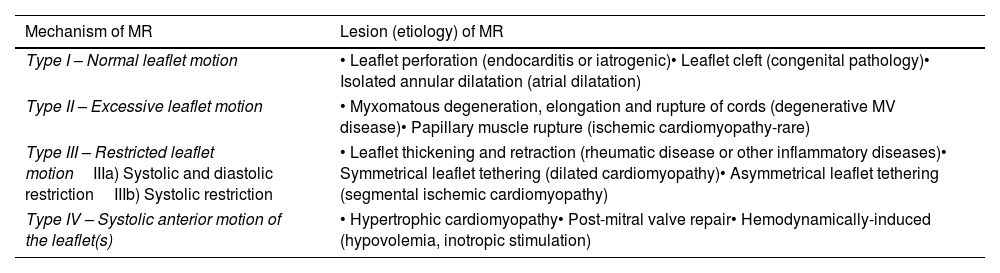
The mechanism of MR is commonly classified on the basis of the leaflet motion using the Carpentier's classification.13 According to this classification, type I MR is defined as normal leaflet motion, type II is characterized by excessive leaflet motion and type III is characterized by restrictive leaflet motion. More recently, an update of this classification has been proposed including the type IV of MR, defined by systolic anterior motion (SAM) of leaflets and typically occuring in patients with hypertrophic cardiomyopathy14 (Table 1).
Classification of mitral regurgitation.
| Mechanism of MR | Lesion (etiology) of MR |
|---|---|
| Type I – Normal leaflet motion | • Leaflet perforation (endocarditis or iatrogenic)• Leaflet cleft (congenital pathology)• Isolated annular dilatation (atrial dilatation) |
| Type II – Excessive leaflet motion | • Myxomatous degeneration, elongation and rupture of cords (degenerative MV disease)• Papillary muscle rupture (ischemic cardiomyopathy-rare) |
| Type III – Restricted leaflet motionIIIa) Systolic and diastolic restrictionIIIb) Systolic restriction | • Leaflet thickening and retraction (rheumatic disease or other inflammatory diseases)• Symmetrical leaflet tethering (dilated cardiomyopathy)• Asymmetrical leaflet tethering (segmental ischemic cardiomyopathy) |
| Type IV – Systolic anterior motion of the leaflet(s) | • Hypertrophic cardiomyopathy• Post-mitral valve repair• Hemodynamically-induced (hypovolemia, inotropic stimulation) |
Moreover, the characterization of valve pathology should be refined by segmental MV analysis, including the assessment of scallops and commissures, which permits precise localization of valve lesion(s) ad may also provide additional information on the etiology. For degenerative MV disease, that represents the leading cause of primary MR in Western countries,2 this analysis can help in identifying two opposite entities: Barlow disease, which is characterized by multi-segmental billowing, redundancy and thickened tissue, and fibroelastic deficiency, where the typical lesion is a chordal rupture with the involvement of a isolate scallop of the posterior leaflet.15
Notably, 3D TEE is particularly useful for a detailed characterization of the location and extent of MV lesion(s)16–19 and demonstrated to be superior for the diagnosis of clefts and commissural lesions, as compared to 2D TEE.17,19 In addition, using commercially available software for the analysis of 3D dataset, the mitral annulus and leaflet can be traced to create 3D model of the mitral valve (Fig. 3B). From these models, highly accurate measurements of mitral annular size and shape, leaflet surface area and papillary muscle location can be obtained.10,17,18,20 This technologies may also provide quantitative information on MV lesions, such as calculating the billowing volume for degenerative mitral valve disease20 and tenting volume for secondary MR.21,22 3D measurements provided insight into the effects of various MV pathologies (i.e. alterations of annular geometry and function occurring in patients with degenerative and secondary MR),23 and may be useful for directing repair techniques.17,18,20 Recently, artificial intelligence has also been combined with the technologies for 3D MV modeling, enabling a semi-automated assessment of MV pathology and showing a better agreement with surgical findings in comparison to manual analysis.24,25
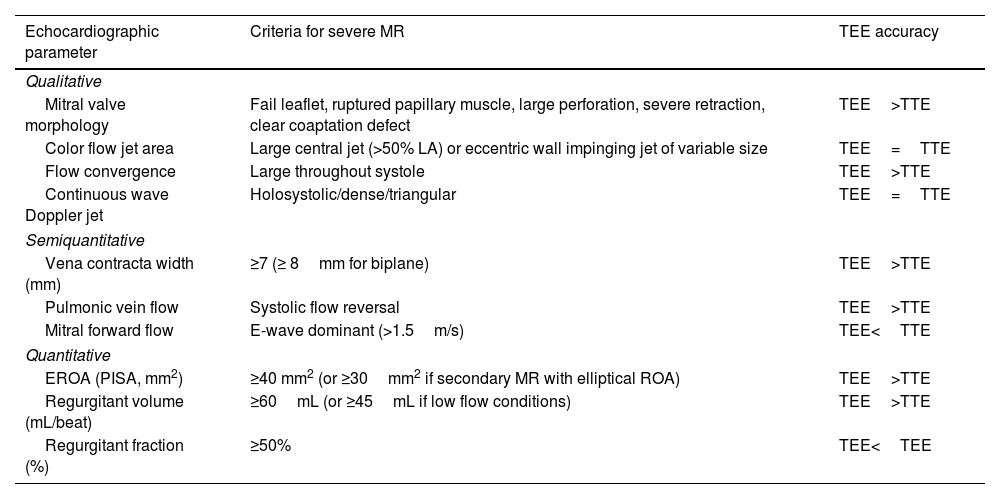
Quantification of mitral regurgitation severityTEE enables an accurate quantification of MR severity and becomes of crucial importance in patients in whom TTE is inconclusive or technically difficult. The criteria for defining severe MR with 2D echocardiography are listed in Table 2.12,26 The majority of the parameters for MR quantification with TTE can be also used during TEE. Particularly, the higher resolution of TEE, the multiplane capabilities and the proximity of the probe to the MV make vena contracta and proximal isovelocity surface area (PISA) imaging easier and more accurate in comparison to TTE measurements.7 Furthermore, interrogation of all pulmonary veins is generally feasible.
Criteria for severe mitral regurgitation based on 2D echocardiography.
| Echocardiographic parameter | Criteria for severe MR | TEE accuracy |
|---|---|---|
| Qualitative | ||
| Mitral valve morphology | Fail leaflet, ruptured papillary muscle, large perforation, severe retraction, clear coaptation defect | TEE>TTE |
| Color flow jet area | Large central jet (>50% LA) or eccentric wall impinging jet of variable size | TEE=TTE |
| Flow convergence | Large throughout systole | TEE>TTE |
| Continuous wave Doppler jet | Holosystolic/dense/triangular | TEE=TTE |
| Semiquantitative | ||
| Vena contracta width (mm) | ≥7 (≥ 8mm for biplane) | TEE>TTE |
| Pulmonic vein flow | Systolic flow reversal | TEE>TTE |
| Mitral forward flow | E-wave dominant (>1.5m/s) | TEE<TTE |
| Quantitative | ||
| EROA (PISA, mm2) | ≥40 mm2 (or ≥30mm2 if secondary MR with elliptical ROA) | TEE>TTE |
| Regurgitant volume (mL/beat) | ≥60mL (or ≥45mL if low flow conditions) | TEE>TTE |
| Regurgitant fraction (%) | ≥50% | TEE<TEE |
Abbreviations. 2D: two-dimensional; EROA: effective regurgitant orifice area; MR: mitral regurgitation; PISA: proximal isovelocity surface; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography.
On the other hand, it is important to acknowledge the potential hemodynamic changes in fluid-depleted and sedated patients during TEE examination, that may lead to underestimation of MR severity. Therefore, blood pressure and heart rate should always be recorded and their effect taken into account when investigating MR.26 In addition, quantitative pulsed Doppler is more challenging with TEE and, particularly, pulsed Doppler interrogation of LV outflow tract is usually limited by angulation issues, leading to underestimation of systemic output (and thereby overestimation of regurgitant fraction).26
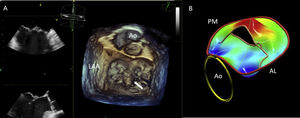
3D color Doppler imaging improves the echocardiographic quantification of MR severity, particularly for the estimation of effective regurgitant orifice area (EROA) and regurgitant volume.10,11 Indeed, the accuracy of 2D PISA method to estimate EROA is limited by the presence of hemi-elliptic shape of the flow convergent region or by the presence eccentric regurgitant jets (given the assumption of hemispherical shape symmetry).27 3D TEE overcomes this limitation of 2D imaging, allowing the direct delineation of vena contracta area or EROA. Direct assessment of 3D vena contracta area has demonstrated that is not circular in the majority of the patients, revealing a prevalent hemi-elliptical shape in patients with secondary MR and a broad spectrum of irregular shapes in patients with degenerative MR28 (Fig. 4). A threshold of 3D vena contracta area of ≥0.41 has been reported as indicative of severe MR,27 but this cut-off requires further validation. Importantly, 3D EROA and regurgitant volume have been shown to correlate more closely with cardiac magnetic resonance measurements, as compared with 2D TEE.29,30 An additional promising method of MR quantification by 3D imaging is the direct delineation of anatomic regurgitant orifice area (AROA) by visualization of the valve en face using parametric maps.31,32 These direct measurements are advantageous since they may account for the nonplanar geometry of the regurgitant orifice and enable the planimetry and then summation of different orifice areas in patients with multiple MR jets, to obtain a more accurate assessment of MR severity.10
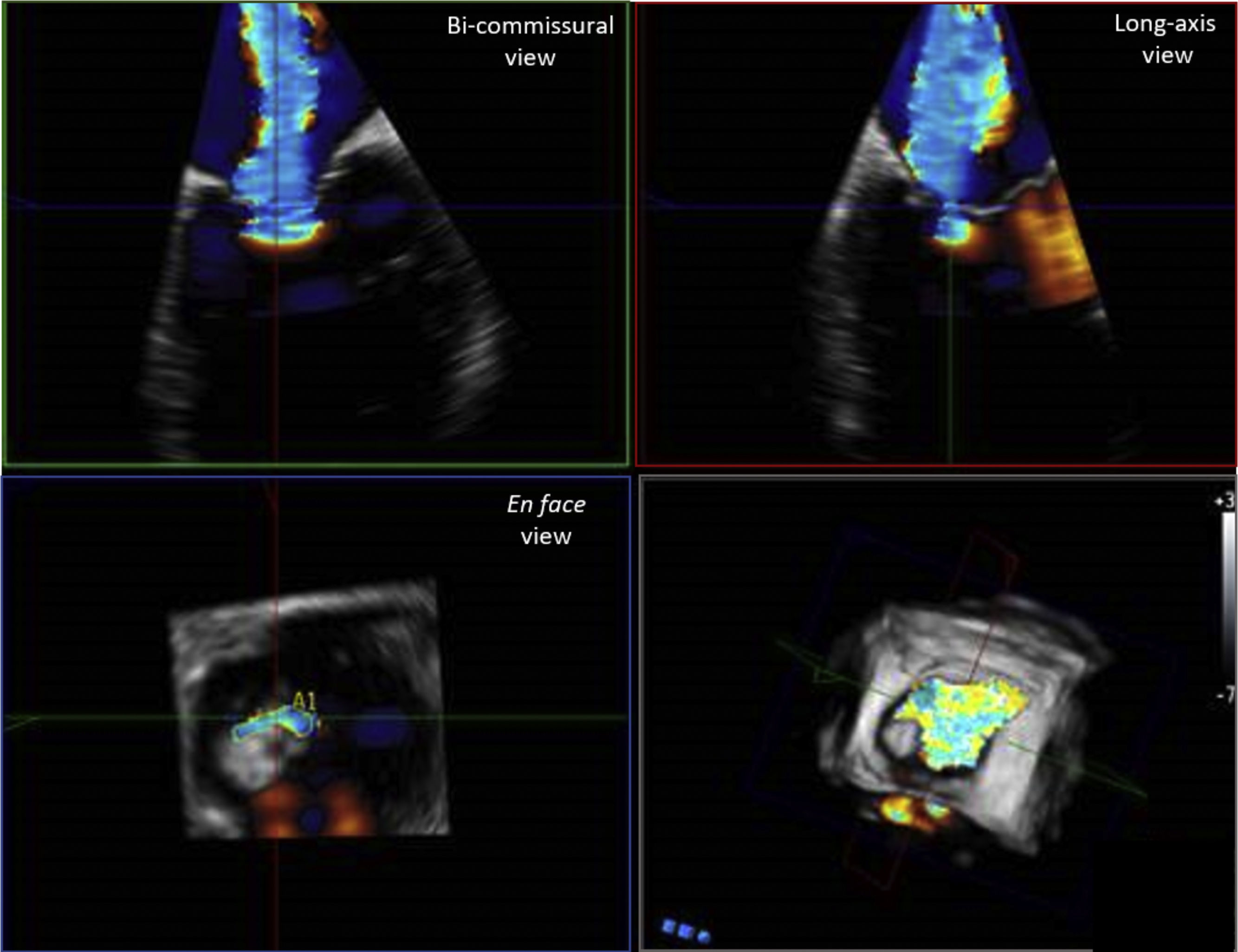
3D echocardiographic technique for the assessment of vena contracta area. Legend. Example of measurement of vena contracta area using 3D commercial software (Q-LAb, Philips Medical Systems, Andover, MA). 3D dataset is manually rotated to parallel the long axis of the jet, in each of the two ortogonal planes. The third plane (en face view from the left ventricle perspective) is set to the narrowest part of the jet, immediately distal to the regurgitant orifice. In this view, vena contracta area can be measured by manual planimetry of the color Doppler signal. In this example of a patient with severe secondary MR, the typical noncircular slit-like vena contracta area along the commisural line is visualized.
Exercise stress echocardiography (SE) may also provide crucial information regarding the severity and the hemodynamic impact of MR. Exercise SE is clinically indicated in patients with severe MR without symptoms and patients with moderate MR with symptoms. In both clinical scenarios, exercise SE enables to identify patients with truly severe MR and symptoms, LV systolic dysfunction or other hemodynamic consequences (i.e. systolic pulmonary arterial pressure rise to>60mmHg), which thereby should be candidates for MV surgery.33
Surgical considerations in primary MRMitral valve repair is the preferred surgical treatment for primary MR, with significant advantages over MV replacement, in terms of long-term survival and quality of life.34,35
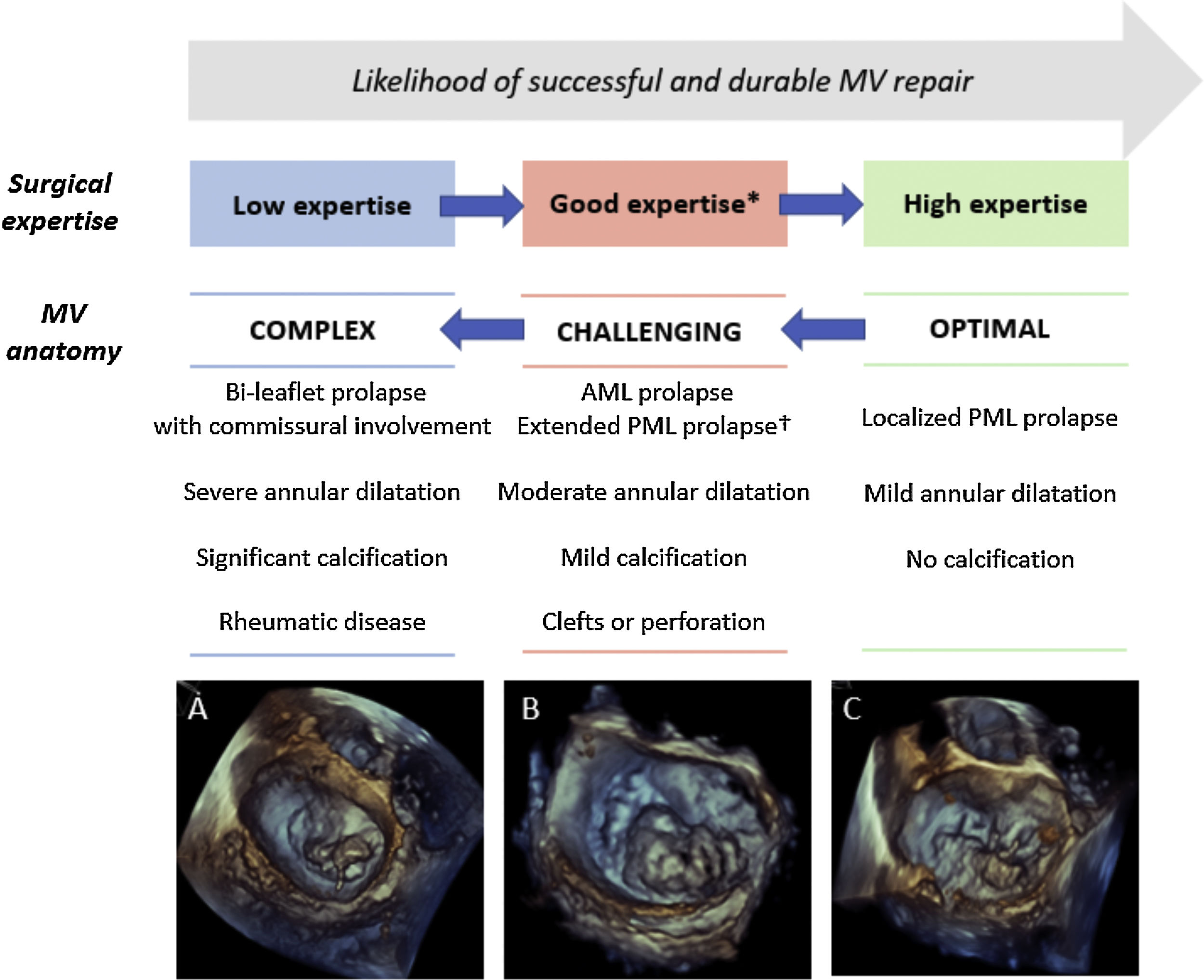
In patients with severe primary MR, the onset of symptoms or signs of LV systolic dysfunction (LVEF<60% or LVESD>40mm) provides a class I indication for surgical intervention, irrespective of whether the valve is repaired or replaced.12,36 Atrial fibrillation and pulmonary hypertension have also been associated with worse outcomes and should be considered as triggers for intervention, regardless of surgical strategy (class IIa indication in the European guidelines on Valvular Heart diseases).12 However, for patients with severe MR that have not reached these surgical thresholds, early MV repair has been shown to potentially restore normal life expectancy.4,5 Therefore, despite no randomized trials have been performed comparing early repair versus watchful waiting strategy, some evidence4,5 supports the potential benefit of an early surgical referral, when durable repair is likely and may be performed in a center with a high level of surgical expertise in valve disease (Heart Valve Center).36 These findings resulted in the indication, provided by the American guidelines,36 to consider early surgical repair in asymptomatic patients with severe MR and no LV dysfunction, if a success rate>95% with an operative mortality<1% can be expected at a given center. Conversely, for this sub-set of patients, the European guidelines suggested a watchful waiting, unless significant LA dilation is present.12 Of importance, the expected success rate for MV repair should be evaluated taking into account the MV anatomy, as well as the surgical expertise.
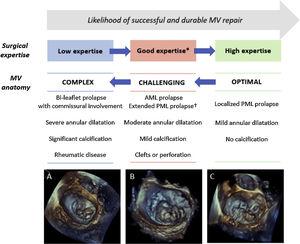
A detailed TEE assessment of MV pathology is crucial for determining the anatomic suitability for MV repair and, then, for planning the surgical techniques. In patients with primary MR, the likelihood of repair decreases with the increase in the complexity of valve lesion(s).37 The valve abnormality that typically is associated with successful repair is fibroelastic deficiency, characterized by chordal rupture involving an isolated scallop of the posterior leaflet (typically P2). In patients with this condition, quadrangular or triangular resection with implantation of an annular ring results in durable repair, achievable by the majority of cardiac surgeons. Increasing complex degenerative anatomy (ranging from P1 or P3 involvement, isolated anterior leaflet prolapse, commissural involvement and bi-leaflet disease), that are commonly observed in patients with Barlow disease, requires additional surgical procedure such as chordal transfer or implantation of artificial chords and therefore a higher level of surgical expertise to achieve a successful repair37 (Fig. 5). 3D imaging may provide additional qualitative and quantitative information, to characterize the complexity of degenerative lesion(s), in comparison to 2D TEE.10 Particularly, 3D volumetric calculation of billowing volume, with a cut-off threshold of >1.15mL, has been reported as discriminating factor between fibroelastic deficiency and Barlow disease and as predictor of surgical repair complexity.18
Assessment of the probability of a successful and durable MV repair in primary MR. Legend. The likelihood of performing a successful and durable MV repair is related to the complexity of MV anatomy and the surgical expertise. Decreasing complexity of MV degenerative anatomy are illustrated: (A) bi-leaflet prolapse with A3 flail and annular calcification, (B) large P2-P3 prolapse (involving>50% of the leaflet surface), (C) isolated P3 flail. * Defined as>25 repairs/year per operator or >50 repairs/year per center. † Defined as involving>50% of the PML. Abbreviations. AML: anterior mitral leaflet; PML: posterior mitral leaflet.
The presence of significant annular or leaflet calcification has also important surgical implications, since it may affect the possibility or the durability of MV repair.37 Additionally, indentation and clefts of the MV leaflets, defined as grooves separating the leaflet scallops with a depth<50% and >50% of the leaflet length respectively, may be the cause of early failure of MV repair.10 During the surgical inspection, clefts and indentations may be difficult to visualize, since the heart is flaccid, especially when the scallops are diseased.10 Accordingly, their identification during the pre-operative TEE assessment, which is significantly magnified by using 3D imaging, is particularly relevant in patients being considered for MV surgical repair.38
Quantitative 2D or 3D measurements of annular and leaflet dimensions are useful for guiding surgical planning of MV repair and, thus, should be reported in the TEE report of patients with severe MR.
On 2D-TEE, mitral annular size is estimated by measuring the antero-posterior diameter (ME long-axis view) and the inter-commissural diameter (ME commissural view) at end-systole. Annular inter-commissural diameter and anterior leaflet length can be used to predict the size of annuloplasty ring or band. However, three-dimensionally derived measurements of annular and leaflet size, particularly inter-trigonal distance (which is typically smaller than the inter-commissural diameter), annular circumference and A2 length, demonstrated a higher agreement with surgical findings and, when available, should be preferred.39,40
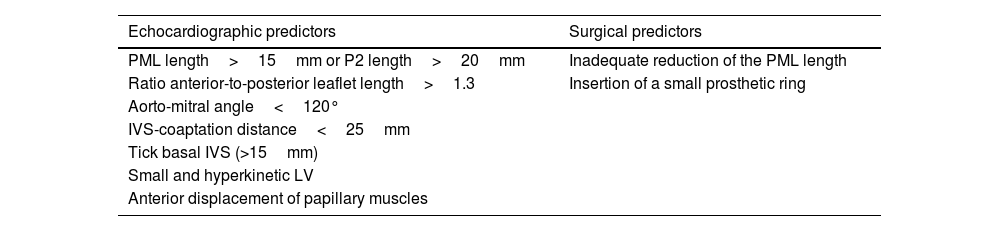
The assessment of the posterior leaflet length is also an important consideration for tailoring the surgical approach. Indeed, a long posterior mitral leaflet (PML), defined by PML length>15mm on 2D imaging, is associated with an increased risk of post-operative systolic anterior leaflet motion (SAM) with LV outflow tract obstruction and, thus, may suggest the need of leaflet resection in order to avoid this complication41,42 (Table 3). Alternatively, P2 leaflet length>20mm on 3D imaging has also been reported as potential determinant of posterior leaflet resection.40
Risk factors of systolic anterior mitral valve motion after mitral valve repair.
| Echocardiographic predictors | Surgical predictors |
|---|---|
| PML length>15mm or P2 length>20mm | Inadequate reduction of the PML length |
| Ratio anterior-to-posterior leaflet length>1.3 | Insertion of a small prosthetic ring |
| Aorto-mitral angle<120° | |
| IVS-coaptation distance<25mm | |
| Tick basal IVS (>15mm) | |
| Small and hyperkinetic LV | |
| Anterior displacement of papillary muscles |
Abbreviations. SAM: systolic anterior motion; IVS: interventricular septum; LV: left ventricle; PML: posterior mitral leaflet.
Mitral valve surgery (repair or replacement) has not been proven to confer survival benefits in secondary MR (caused by LV disease), although it may improve symptoms and quality of life.43,44 Therefore, current surgical indications are restricted to patients with severe MR undergoing coronary revascularization or other cardiac surgery and patients with severe MR, who remain symptomatic despite optimal medical therapy (including cardiac resynchronization therapy if indicated).12,36
For patients with secondary MR, a detailed echocardiographic assessment of LV remodeling (LV volumes, ejection fraction, and sphericity index) and geometric MV distortion (tenting area, leaflet coaptation depth, leaflet angles, and inter-papillary muscle distance) is mandatory for defying the more appropriate surgical approach. Indeed, in patients without advanced LV remodeling and severe leaflet tethering, mitral valve repair with an undersized complete ring may offer satisfactory results.45 Conversely, in patients with echocardiographic predictors of annuloplasty failure46–48 (Table 4), additional valvular/subvalvular techniques or chordal sparing valve replacement should be considered to achieve a more durable solution.49 Nevertheless, it is important to acknowledge that no one of these singular echocardiographic parameters demonstrated a strong and highly reproducible association with annuloplasty failure, while discrepant results have been reported by different studies on this topic.50,51 With this regard, it has been suggested that 3D imaging may significantly improve the value of pre-operative TEE for predicting annuloplasty outcomes.51 Accordingly, the P3 tethering angle (with a threshold value of 30° or higher) has been reported as stronger predictor of ischemic MR recurrence, in comparison to 2D posterior tethering angle.51 In addition, 3D MV parametric maps enable an accurate reconstruction of tenting volume shape and quantification of tenting volume.52,53 Tenting volume was closely related to MR severity in patients with ischemic cardiomyopathy, despite its potential value for predicting annuloplasty failure has not been determined.53
2D echocardiographic characteristics identifying unfavorable outcome to undersized annuloplasty in patients with secondary mitral regurgitation.
| Parameters of MV geometry |
| Leaflet coaptation depth>10mm |
| Systolic tenting area>2.5–3cm2 |
| Posterior mitral leaflet angle>45° |
| Distal anterior mitral leaflet angle>45° |
| Parameters of global or regional LV remodeling |
| Global LV remodeling: LVEDD>65mm, LVESD>51mm |
| Systolic interpapillary muscle distance>20mm |
| Systolic sphericity index>0.7 |
| Basal aneurysm/dyskinesia |
Abbreviations. 2D: two-dimensional; LV: left ventricular; LVEDD: LV end-diastolic diameter, LVESV: LV end-systolic diameter.
In patients with atrial functional MR, mitral annular dilatation, caused by severe atrial remodeling, represents the main pathogenetic mechanism (type I), in absence of LV disease by definition.12 Thereby, this sub-group of patients may be more effectively treated by a therapeutic approach targeting the annulus (MV annuloplasty, eventually associated with atrial fibrillation ablation), in comparison to patients with secondary MR, for which the sub-valvular tethering is the key determinant of valve dysfunction (type III). Accordingly, some observational studies described better outcomes in patients with atrial functional MR treated by MV annuloplasty,54 but the evidence is still limited and current guidelines did not provide specific indications for surgical referral in this population.12
ConclusionsQualitative and quantitative date obtained by 2D and 3D TEE provide critical insights into the morphological and functional MV abnormalities, which are essential for defining the appropriate time of intervention and for tailoring the surgical approach. Newer 3D technologies, such as parametric MV modeling, showed promising results in further improving the patient’ selection, planning and prediction of outcomes for surgical MV repair.
Ethical considerationsThis article has not involved animals or humans, does not relate to a clinical trial and all the data shown in figures and tables are appropriately indicated in the main text of the review.
Conflict of interestThe department of Cardiology, Heart Lung Center, Leiden University Medical Center, received research grants from Abbott Vascular, Bayer, Bioventrix, Medtronic, Biotronik, Boston Scientific, GE Healthcare and Edwards Lifesciences. Victoria Delgado received speaker fees from Abbott Vascular, Medtronic, Edwards Lifesciences, Novartis, MSD and GE Healthcare.