The present article is the first official report of ESPAMACS (Spanish Registry for Mechanical Circulatory Support) and summarises the registry activity from when it began operating in October 2014–May 2016. During this period, 369 mechanical circulatory support devices, implanted in 18 different centres of our country have been registered, 319 for short-term support (86.4%), and 50 for long-term support (13.6%). An analysis is presented of the profile of the assisted patients (demographic data, comorbidities, underlying disease, grade of heart failure), type of implanted devices, indications, surgical data and outcomes (post-operative outcome, duration of support, level of achieving objectives, adverse events, survival, and causes of death).
Este artículo representa el primer informe oficial de ESPAMACS (Registro Nacional Español de Asistencia Mecánica Circulatoria) y en él se expone la actividad del registro desde que comenzó a funcionar en octubre de 2014 hasta mayo de 2016. A lo largo de este periodo se han registrado 369 dispositivos de asistencia mecánica circulatoria implantados en 18 centros de nuestro país, 319 de corta duración (86.4%) y 50 de larga duración (13.6%). Se analiza el perfil de los pacientes (datos demográficos, comorbilidades, enfermedad de base, grado de insuficiencia cardiaca), el tipo de dispositivos implantados, las indicaciones, los datos operatorios y los resultados (evolución postoperatoria, duración del soporte, grado de consecución de objetivos, efectos adversos, supervivencia y causas de muerte).
Considering the technological developments in mechanical circulatory support (MCS) for patients with end-stage heart failure, no clinician would doubt the need to register the events and data emerging from the application of this therapy. Information about the events in individual patients, as well as the statistical evaluation of different groups of patients and the information about the functioning of devices over time can reveal a lot about the safety of the applied therapy, freedom of adverse events and survival. Such data lead to adaptation of clinical practice based on the registered outcomes, and may result in new possibilities for improvement and/or in technical innovation. Physicians may use data for patient selection and the development of bespoke treatment strategies. Moreover, based on these data, the information to patients and their next of kin about the expected outcomes of MCS therapy may become more accurate. The database offers individual hospitals a tool for benchmarking, while researchers may correlate data to gain science-based insights and define factors that influence patient care and outcomes. The registry enables other stakeholders, such as the industry that produces the devices, to use data to initiate innovations, and to measure the results of those.
RetrospectiveAs history shows, local registries, frequently developed by the treating physician(s) or their in-hospital ICT (Information and Communication Technology) departments, became the source for professionals to demonstrate their local achievements. Then, those who think big, and rightly so, develop initiatives to gather data on a larger geographical scale. While in the USA hospitals were obliged to provide data to INTERMACS, the Europeans created a voluntary registry, called EUROMACS, which connects with local and national databases. After an initiative of Prof. Roland Hetzer, EUROMACS was founded in 2009 as a non-profit association that functions as a Committee of the European Association for Cardio-Thoracic Surgery. Forty-seven hospitals from 16 countries have now joined, and another 20 are taking steps to follow. The difference between EUROMACS and other registries is that EUROMACS provides data to professionals who wish to carry out clinical and/or scientific analyses. Further, data completeness checks by statisticians and on site audits add to the quality of the data. The development of a near real-time dashboard will enable the participating sites to benchmark their outcomes by comparison with the anonymous data of the other hospitals.
For reasons of different regulatory environments and spans of control, an agreement with the IMACS Registry sees to the provision of data on a global level. Thus we have connected the world of MCS from the ground up, from local to global, expecting that the clinical and scientific data will enable us to learn how to improve the care of patients with end-stage heart failure.
EUROMACS organization- A.
Structure
As a Committee of the EACTS, EUROMACS is democratically structured. Members of the executive board, which have maximal five members, are elected by the members for a period of three years with a possibility to be re-elected for another three years. The extended board has a maximum of seven members and serves to reflect the diversity in nationalities of participants in the Registry. Extended board members are also chosen for a period of maximal three years, twice. While the executive board sees to the execution of the aims of the organization, the extended board approves the strategic planning, the annual report and financial report before it is sent to the members. The executive board decided to appoint a managing director who manages the day-to-day business of the organization and facilitates the work of the board.
- B.
Providing data to clinicians and scientists
The first aim of the EUROMACS registry is to provide data to the community of clinicians and scientists.
- 1.
On a regular basis (Annual Report)
- 2.
Via the EUROMACS dashboard, after login
- 3.
For scientific studies
- 4.
Bespoke. E.g. for (national) statistics, for use on congresses.
The first annual report was published in March 2015.1 The second report is expected in the summer of 2016.
Contributors of data, after they entered the EUROMACS registry by means of their password, will immediately see the possibility to open a dashboard with statistics. These statistics not only provide general information about the number of cases in the registry, it also shows comparisons between the data from the hospital of the user with the entire EUROMACS database. Further, any clinician or scientist can submit a study proposal to the EUROMACS Committee, and request anonymous data from the Registry. The applicant is asked to agree to use the data for the sole purpose of the study they were asked for. The data from EUROMACS were used for several studies and publications.2
- 1.
- C.
Collection of data
A hospital that wishes to participate in the EUROMACS Registry is offered an agreement in which it accepts to provide pseudo-anonymised base-line and follow-up data to the registry. A unique password allows the responsible physician and/or data manager to enter patient records and events. Three methods are at the disposal of the participants:
- a)
Submitting the data patient per patient. This method is appropriate for hospitals that have a relatively low number of implants per year.
- b)
Uploading from the local database. This method fits hospitals that historically have a local database in which they register the details of the treatment of MCS patients. The advantage is that uploading these data avoids double data entry.
- c)
Regular data transfer from a national database, by means of a unique secure link, to EUROMACS.
The EUROMACS data set consists of several groups:
- •
Base-line data such as sex, age, primary diagnosis, laboratory data and blood circulation data before MCS implantation. The number of mandatory data is limited to seven.
- •
Quality of life data (EQ 5D).
- •
Peri-operative data.
- •
Any event after the implantation. A distinction is made between major events and others, while routine follow-up is registered when the patient has come in for a check-up. The number of mandatory data is six.
The hospitals participating in the EUROMACS registry have agreed to register events within 6 weeks after their occurrence. Every six months, per June 30, and per December 31, they are asked to confirm that their data are up-to-date. The EUROMACS management offers them an overview of their patients and, if this would be the case, a break-down of missing data. After completion and correction the data are consolidated, from then on they can be made available for analyses and studies.
- a)
- D.
Quality control
Quality control, as referred to in the previous paragraph, consists of the statistical analysis of the data submitted by the participating centers. An overview of missing or inconsistent data is provided to the centers every six months. If data would be faulty, or missing, the centers are invited to correct the non-compliance.
On-site audits are a second instrument to guarantee that data collection and registration in the database reflects the reality in the participating clinics.
- E.
Global cooperation
In 2013, EUROMACS and IMACS signed an agreement. The agreement sees to an annual anonymous data transfer from EUROMACS to IMACS. Whereas IMACS’ function is to gather data on MCS implants on a global level, EUROMACS focuses on information from hospitals in countries that are geographically, partly or entirely on the European continent.
The first IMACS report was published early 2016, in the Journal for Heart and Lung Transplantation.3
- F.
Outcomes
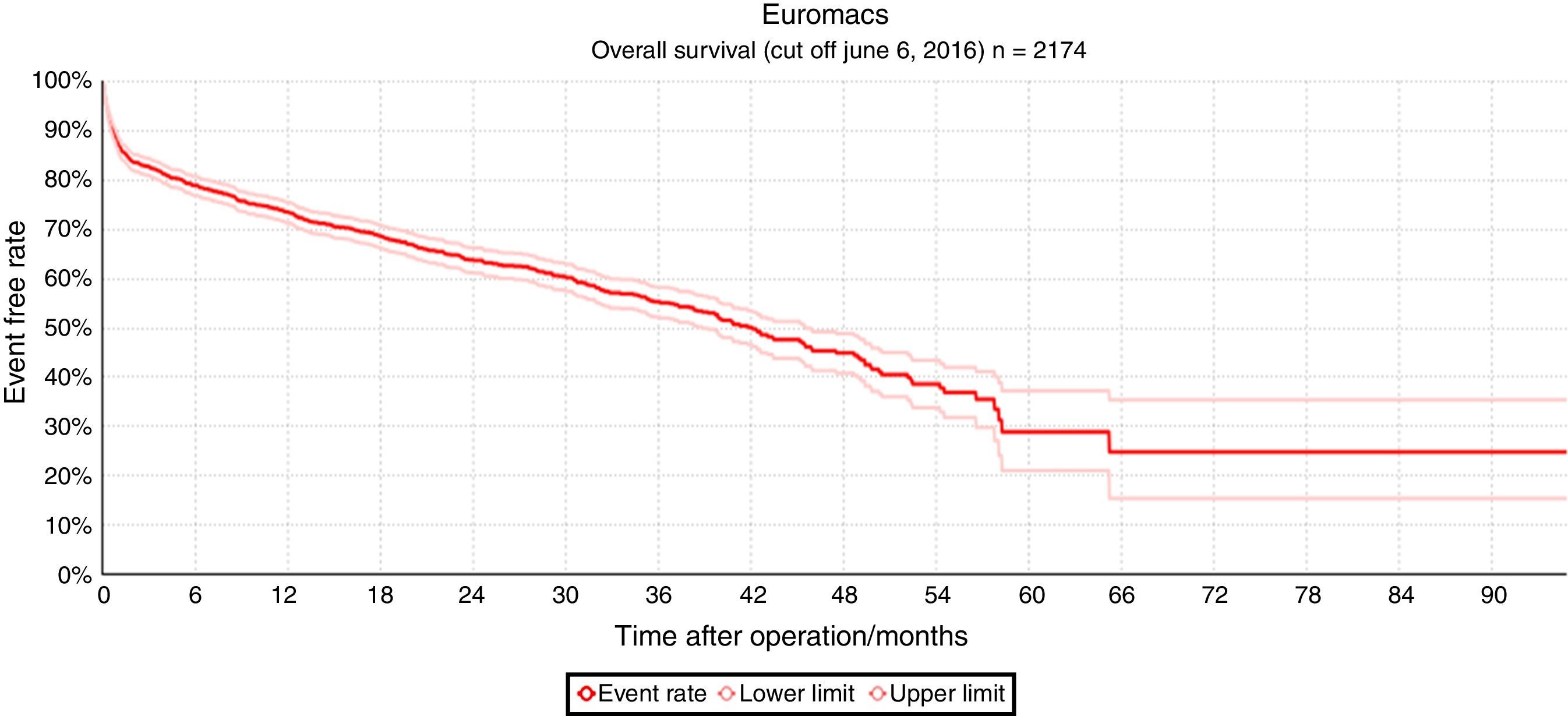
Fig. 1 shows the result of all MCS implantations registered in EUROMACS, in which the overall actuarial survival outcomes of 2174 primary implantations registered through June 6, 2016 are depicted.
The overall actuarial survival of 2172 MCS patients after 6 months, 1, 2, 3, and 4 years was 78.3%, 72.9%, 62.3%, 54.9% and 44.7%, respectively. Patients at risk were 498 (at 2 years), 268 (at 3 years), and 100 (at 4 years).
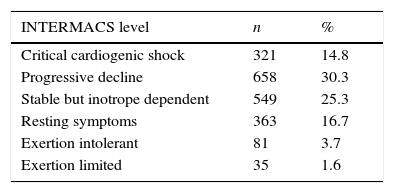
Table 1 provides a break down of the INTERMACS levels of the MCS patients in the EUROMACS registry. The EUROMACS database shows that 55.6% of patients with MCS were in INTERMACS profiles 2 and 3, whereas the seventh INTERMACS report showed a total of 66.3% of patients in these two levels.4 In comparison with the same INTERMACS report, wherein 15% of patients were in critical cardiogenic shock at the time of implantation, the EUROMACS database showed comparable 14.8% (see Table 1) of patients in this category.
Table 1.INTERMACS profiles of 2172 VAD implantations.
INTERMACS level n % Critical cardiogenic shock 321 14.8 Progressive decline 658 30.3 Stable but inotrope dependent 549 25.3 Resting symptoms 363 16.7 Exertion intolerant 81 3.7 Exertion limited 35 1.6 NYHA, New York Heart Association; VAD, ventricular assist device.
Early 2016, 47 hospitals from 17 countries contributed to the EUROMACS registry. The registered implantations exceeded 2,100, while the number of registered routine follow-up records and “events”, such as transplantations, adverse events, pump exchange and death, was more than 10,000. In the spring of 2016, a connection with the national MCS registry of Spain, ESPAMACS, could be established. Thus, participants in ESPAMACS have access to data on a European level. This access enables them to benchmark their results, as well a possibility to retrieve data for scientific and/or clinical analyses.
As more individual hospitals and national databases such as the French, the Polish and the Dutch join the Register, it is expected that the number of registered implantations will be more than 4000 by 2017.
Starting in the summer of 2016, software to execute additional statistical analyses, such as frequency of follow-up, ‘near real-time’ benchmarking of survival statistics and freedom of adverse events comparisons will be introduced.
As part of the EACTS, the EUROMACS Registry will be able to benefit from the technology that is being developed within the framework of the EACTS QUIP Project. This technology will open up new statistical pathways and interactive tools which enable e.g. risk assessment and create possibilities for participants to diversify their benchmarking according to patient morbidity, implant site and size of the MCS program.
ConclusionThe EUROMACS database provides a multi-functional tool for cardio-thoracic surgeons, cardiologists and other professionals who are engaged with providing care to patients with MCS. Registration in itself gives insight in the quantitative aspects of the therapy. While some hospitals use EUROMACS to keep track of their own implantations and follow-up, others have the objective to compare their outcomes with those of all participating centers. Likewise, national societies, such as the Spanish Society of Thoracic-Cardiovascular Surgery (SECTCV) or the French SFCTCV, may use EUROMACS as a national database enabling measuring the performance of all MCS programs in the country either individually or collectively. The link with EUROMACS, and on its turn, the links with the EACTS and IMACS, enable all participants to use software and data with which they can benchmark themselves. The tools offered make it possible to identify strengths and weaknesses of the outcomes per hospital, or from a group of hospitals (if all those in that group agree), and to focus on improvements where necessary. It is expected that, over time, the available data will contribute to beneficial results for patients with MCS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FundingEUROMACS is funded by the European Association for Cardio Thoracic Surgery (EACTS), the Friede-Springer-Herz-Stiftung, and by donations of CircuLite, HeartWare, Inc., Thoratec Corporation, Syncardia and Micromed. These institutions had no influence on study design, collection, analysis and interpretation of data.
Conflict of interestThe authors declare no conflict of interest.
We thank all institutional and individual members of EUROMACS for their continuous support and contributions.