A univentricular (UV) heart is found when both atria drain mainly into one ventricle; while if either ventricle cannot sustain the systemic or the pulmonary circulation, a functionally UV heart emerges. UV heart is the source of hot debates and discussions for decades that stem from controversies in terminologies, analysis, and management.
ObjectiveTo study the morphological pattern of univentricular hearts.
DesignCohort, descriptive study.
SettingReview 13-year data from the file system of a high-volume, tertiary, teaching cardiac centre and study the patients having pathologies meeting the definition of UV heart.
Results461 patients have UV hearts; constituting 1.4% of all patients with a congenital heart disease (CHD) visiting the centre of the study. Functionally UV heart in the form of biventricular atrioventricular (AV) connection plus one ventriculoarterial (VA) connection was seen in 179 cases (38%) with aortic atresia being the most common (N=98; 55%). On the other hand, UV AV connection was seen in 282 cases (62%). Under this category, 203 cases (72%) show an absent AV connection and 79 hearts (28%) with double-inlet anatomy.
ConclusionUV heart is a rare complex CHD with lots of scientific debates. It is hoped that this long-term study will be the basis and a reference to paediatric cardiologists, echocardiographers, and other interested professionals.
Un corazón univentricular (UV) se encuentra cuando ambas aurículas drenan principalmente en un ventrículo; mientras que, si el ventrículo no puede sostener la circulación sistémica o pulmonar, emerge un corazón funcionalmente UV. El corazón UV es la fuente de acalorados debates y discusiones durante décadas que derivan de controversias en terminología, análisis y gestión.
ObjetivoEstudiar el patrón morfológico de los corazones UV.
DiseñoEstudio descriptivo de cohorte.
EscenarioRevisar los datos de 13 años del sistema de archivos de un centro cardíaco docente de alto volumen y terciario, y estudiar a los pacientes con enfermedades que cumplen con la definición de corazón UV.
ResultadosCuatrocientos sesenta y un pacientes tienen corazones UV; constituye el 1,4% de todos los pacientes con cardiopatía congénita (congenital heart disease [CHD]) que visitan el centro del estudio. El corazón funcionalmente UV en forma de conexión biventricular auriculoventricular (AV) más una conexión ventriculoarterial (VA) se observó en 179 casos (38%) y la atresia aórtica fue la más común (N=98; 55%). Por otro lado, la conexión UV/AV se observó en 282 casos (62%). En esta categoría, 203 casos (72%) muestran una conexión AV ausente y 79 corazones (28%) con anatomía de doble entrada.
ConclusiónEl corazón UV es una rara CHD, además de compleja, con muchos debates científicos. Se espera que este estudio a largo plazo sea la base y una referencia para los cardiólogos, ecocardiografistas y otros profesionales interesados en pediatría.
The segmental approach, applied for the analysis of the cardiac malformation, has improved the diagnosis and categorization of the complex congenital heart disease (CHD).
Van Praagh1–3 first highlighted the merits to consider the malformed heart in terms of arterial, ventricular, and atrial components. Some may regard the heart as a “three-floor-complex” – with the ventricles as the first floor, aorta and pulmonary artery (PA) as the second one, and the atria being the platform – connected together through valve orifices and divided by septa.4,5
The three segments are joined by two connections, called atrioventricular (AV) and ventriculoarterial (VA). AV junction connects the atria with their corresponding ventricles and the VA one connects the ventricles with their great arteries. In a normal heart, each junction has two different valves: tricuspid and mitral at the AV and aortic and pulmonary in the VA junction.4
The AV connection is said to be biventricular if each atrium joins on ventricle. When the right atrium (RA) joins the right ventricle (RV), and the left atrium (LA) with the left ventricle (LV), the connection is termed concordant. Further, a discordant connection is found when the RA joins the LV, and the LA is connected to the RV.4
However, sometimes the AV connection cannot be determined as discordant or concordant in the presence of left or right atrial isomerism. In this setting, the topological configuration of the ventricles (i.e. L- or D-loop) must be added.6
A univentricular heart is found when both atria are connected mainly with one ventricle due to one of two possibilities: 1. Double-inlet connection: the presence of single common AV valve or two separate valves that drain predominantly into one ventricle. 2. Absent connection: one of the AV valves is atretic or absent. This leads to the formation of two different ventricles, one is small and hypoplastic and the other is of good size. The latter could have left or right morphological pattern or, on rare occasions, indeterminate. Determining the type of the ventricular loop is an essential step because an absent AV connection might be seen with a discordant loop.
At the VA connection, the anatomy is discordant when the aorta arises from the RV and the PA from the LV, or concordant when the great arteries take origin from their corresponding ventricles.6
Double-outlet VA connection may be seen with one and a half (or more) of the great vessels originate from one ventricle. The single-outlet connection is present when only one patent great vessel arises from the heart, e.g. single pulmonary outlet with atretic aortic valve, a single aortic outlet with atretic pulmonary valve, or single outlet with persistent truncus arteriosus (PTA).
The univentricular hearts are, therefore, the result of univentricular AV connections with resulting one large and another hypoplastic ventricle. Nevertheless, there are hearts with normal AV and VA connections in which one ventricle cannot sustain the systemic or the pulmonary circulation. This is the case of aortic stenosis/atresia causing hypoplastic LV or pulmonary stenosis/atresia, or with Ebstein malformation of the tricuspid valve leading to hypoplastic RV.
Many researchers7–11 unified different anatomical cardiac malformation under the term functionally univentricular heart which is characterised by the fact that either ventricle cannot sustain the systemic or the pulmonary circuit because of its diminutive size or deficient function. In this scenario, the heart is not a candidate for biventricular repair; instead, single-ventricle or one-and-half ventricle repair is feasible.
In this study, we reviewed our 13-year data collection with the aim to identify the morphological characteristics of the hearts fulfilling the new concept of univentricular heart.
Patients and methodsIn this “descriptive cohort” study, we reviewed the file system of a high-volume, busy, tertiary, teaching cardiac centre in Baghdad-Iraq (namely, Ibn Al-Bittar Centre for Cardiac Surgery). We examined all the files made between January 2004 and December 2016 (i.e. 13 years) and identified the patients having cardiac malformations corresponding to the definition of univentricular hearts. A univentricular heart is defined by the fact that both atria are connected mainly with one ventricle due to one of two possibilities: double-inlet or absent connection.6 Cases with a functionally univentricular heart were included. The latter is identified when either ventricle cannot sustain the systemic or the pulmonary circuit because of its diminutive size or deficient function.7–11
For each patient, we recorded the following echocardiographic findings (when available): cardiac position, atrial situs, the morphology of the ventricles, great arteries anatomy and relation, AV and VA connections, and associated anomalies. Each ventricle is regarded as having left, right or indeterminate morphology and being hypoplastic or dominant depending on its size. D- and L-looping of the ventricles were identified. D-loop topology represents a morphological RV in a right-anterior location and the morphological LV in a left-posterior location. On the other hand, L-loop is found when the morphological RV is in the left-anterior and the LV in a right-posterior position.
The nature of the AV valves was identified; whether there is two patent valves, single (common) valve, atretic valve, overriding or straddling valves. Straddling is present when the tensor apparatus (i.e. chordae tendineae and papillary muscles) is inserted – through a VSD – into the contralateral ventricle. While overriding is considered to be present when there is a commitment of the annulus of the AV valve to two ventricular chambers.12
Echocardiography was done using GE Vivid 3 machine (GE Healthcare, USA) in the period between 2004 and 2010; then GE Vivid E9 machine (GE Healthcare, USA) for the remaining period of the study. Two kinds of ultrasonic transducers were used – 3.0 and 5.0MHz.
We reviewed cardiac catheterization data (when available) for those patients with univentricular hearts. Haemodynamics, O2 saturation, and angiographic information were recorded. Different operators have performed the echocardiographic and catheterization studies.
No necropsy (pathological) specimens have been included in this study.
ResultsDuring the period of interest (2004–2016), we found 31,205 newly-diagnosed cases with CHD visited the centre of the study. Cases that met the definition of the univentricular heart were seen in 461 patients (1.4%).
We identified two sets of hearts: first, those with biventricular AV connection plus one VA connection. Second, hearts with univentricular AV connection.
Biventricular AV connection plus one VA connection: 179 casesIn this study, 98 patients (55%) were found having atresia of the aortic valve with intact ventricular septum (IVS), and 81 (45%) with atresia of the pulmonary valve with IVS. Patients with VSD and either aortic (143 cases) or pulmonary atresia (232 cases) were excluded because they often show good-sized ventricles.
All cases of aortic or pulmonary atresia have concordant AV connections and situs solitus.
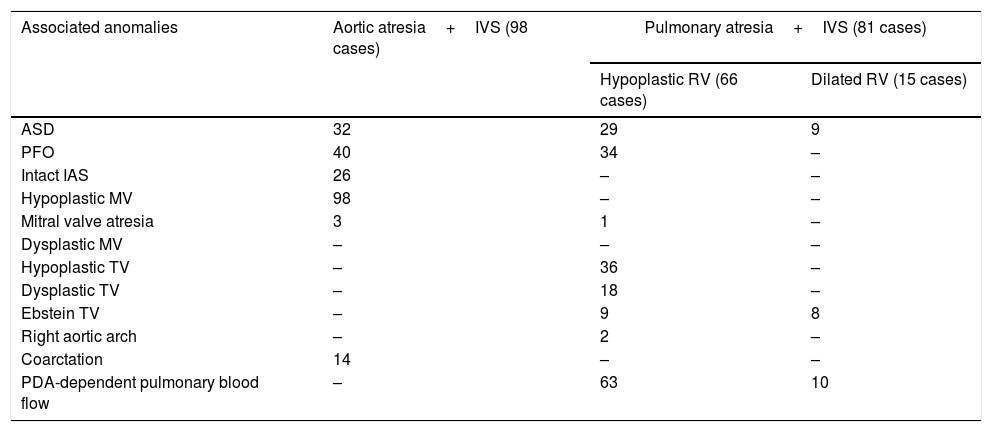
Aortic atresia: 98 casesIn all cases with aortic atresia and IVS, there was D-loop topology of ventricles and levocardia. Associated anomalies are seen in Table 1.
Hearts with biventricular AV connections and single VA connection: associated anomalies.
| Associated anomalies | Aortic atresia+IVS (98 cases) | Pulmonary atresia+IVS (81 cases) | |
|---|---|---|---|
| Hypoplastic RV (66 cases) | Dilated RV (15 cases) | ||
| ASD | 32 | 29 | 9 |
| PFO | 40 | 34 | – |
| Intact IAS | 26 | – | – |
| Hypoplastic MV | 98 | – | – |
| Mitral valve atresia | 3 | 1 | – |
| Dysplastic MV | – | – | – |
| Hypoplastic TV | – | 36 | – |
| Dysplastic TV | – | 18 | – |
| Ebstein TV | – | 9 | 8 |
| Right aortic arch | – | 2 | – |
| Coarctation | 14 | – | – |
| PDA-dependent pulmonary blood flow | – | 63 | 10 |
Abbreviations: ASD, atrial septal defect; IAS, interatrial septum; IVS, Intact ventricular septum; MV, mitral valve; PDA, patent ductus arteriosus; PFO, patent foramen ovale; RV, right ventricle; TV, tricuspid valve.
In all of them, D-loop topology of ventricles and levocardia were seen.
We identified two scenarios: one with dilated, poorly-functioning RV, and another one with a hypertrophied, hypoplastic ventricle.
Hearts with dilated RV: 15 casesAll the 15 cases (18.5%) are presented with dilated RV. The pulmonary valve was imperforate in six cases (40%), absent in three (20%), and not mentioned in the remaining six (40%). Tricuspid valve Ebstein anomaly was seen in 11 cases (73%). Associated anomalies are seen in Table 1.
Heart with hypertrophied RV: 66 casesAmong the 66 cases (81.5%) with the hypertrophied RV, only 20 cases (30%) showed all the three components of the RV (i.e. inlet, trabecular, and outlet). In 30 cases (45%) both outlet and trabecular portions were not seen. In 11 cases (17%) the trabecular portion was not seen, and in the remaining five cases (8%), RV anatomy was not described well.
The pulmonary valve was imperforate in 30 (46%) or the orifice absent in 32 cases (48%) and not mentioned at all in the remaining four (6%).
Hypoplasia of the tricuspid valve is seen in 40 cases (61%), abnormal or dysplastic in 21 cases (32%) and showed Ebstein changes in five (8%).
Connections between the coronaries and ventricle were identified in seven cases (11%). Other associated anomalies are seen in Table 1.
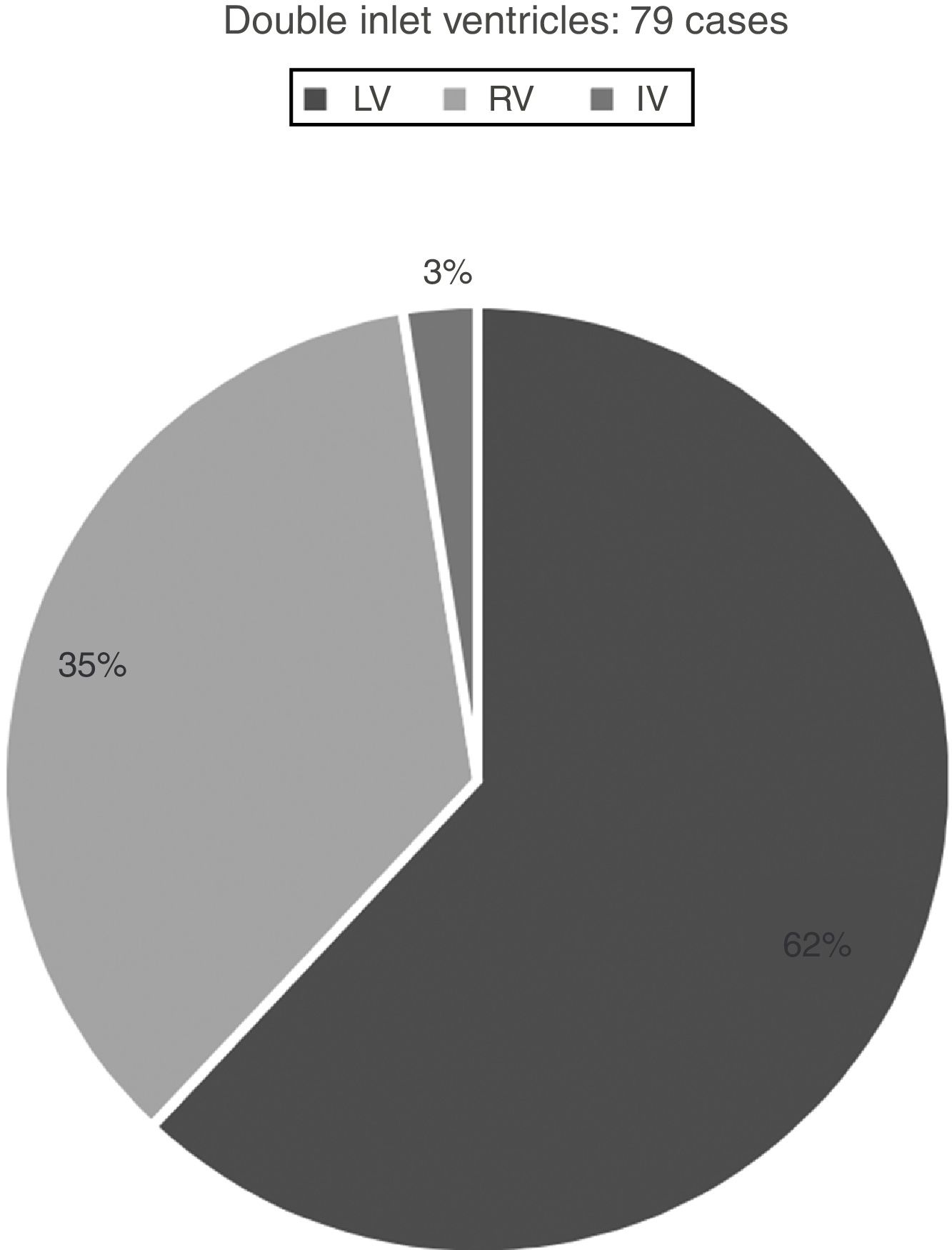
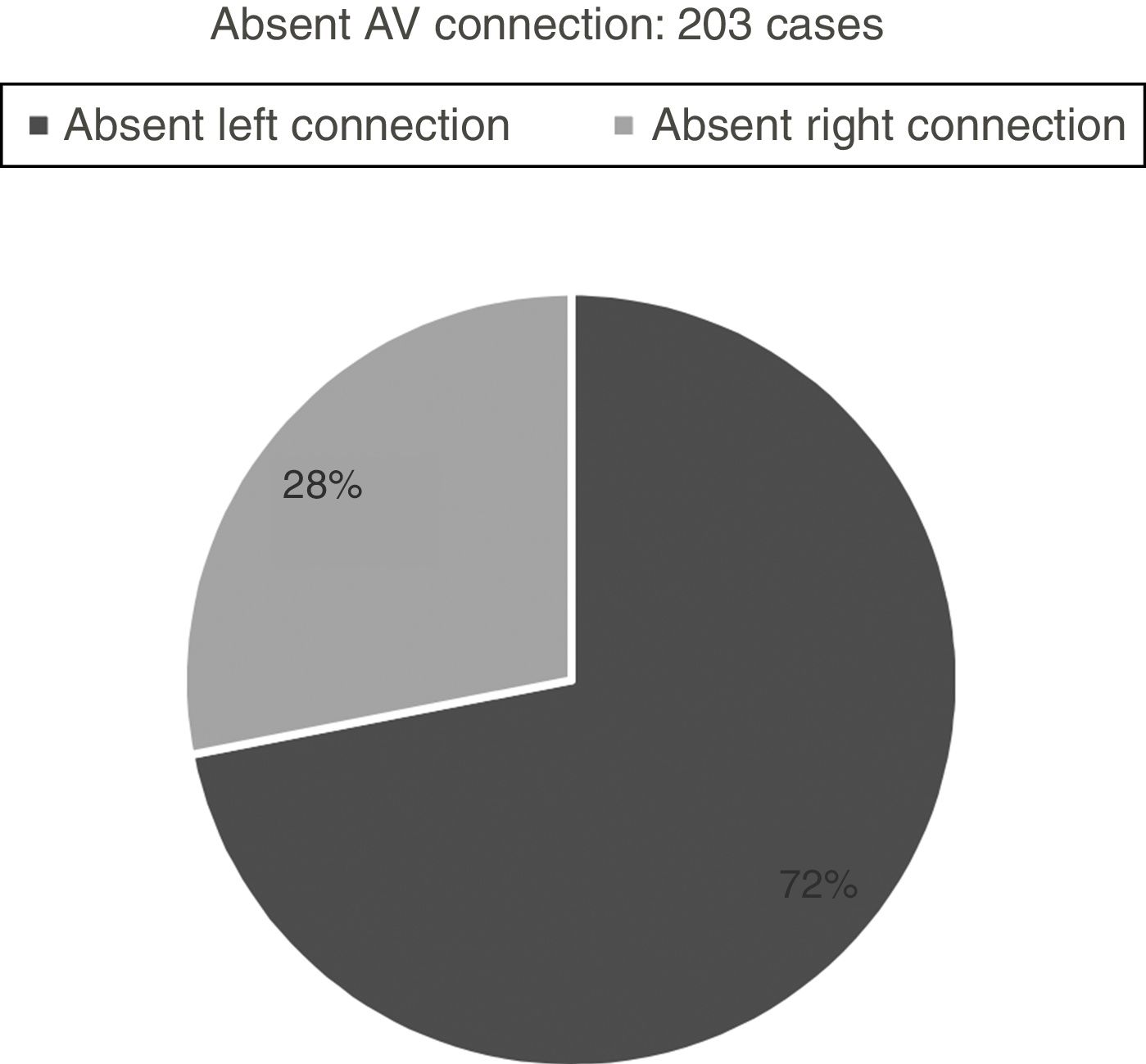
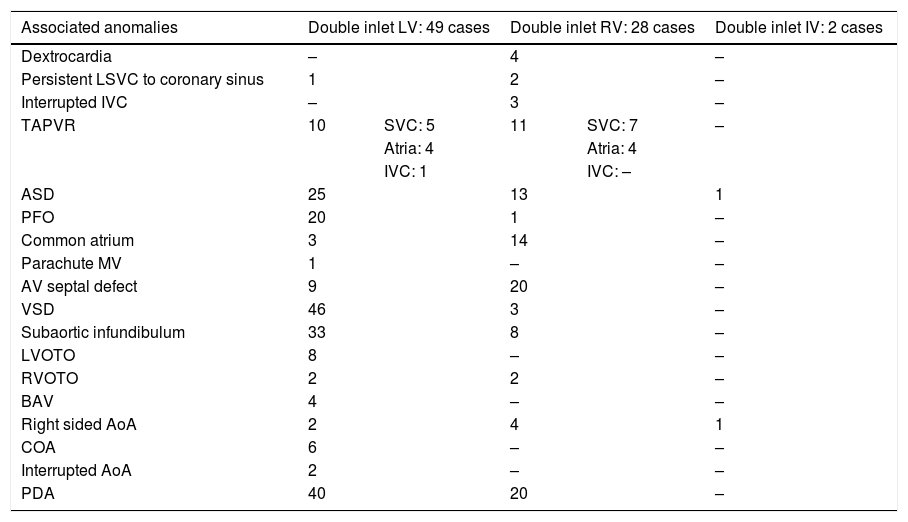
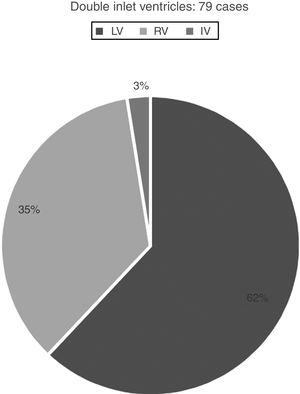
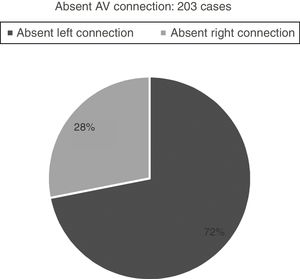
Hearts showing univentricular AV connection: 282 casesWe identified univentricular AV connection in 282 cases. Two-hundred and three cases (72%) showed an absent AV connection and 79 cases (28%) with double inlet connection (Figs. 1 and 2).
Those 79 cases with double inlet connection showed LV dominance in 49 cases (62%), RV in 28 cases (35%), and indeterminate morphology in two cases (3%) (Fig. 1).
All cases with double inlet have situs solitus.
Double-inlet LV (DILV): 49 casesIn double-inlet LV (DILV), two separate patent AV valves were seen in 38 cases. Other variations are seen in Table 2.
Double inlet ventricle: mode of AV connection.
| Mode of AV connection | Double inlet LV: 49 cases | Double inlet RV: 28 cases | Double inlet IV: 2 cases |
|---|---|---|---|
| Two patent valves | 38 | 5 | 2 |
| Common valve | 7 | 23 | – |
| Imperforate right valve | 2 | – | – |
| Imperforate left valve | 2 | – | – |
Abbreviations: AV, atrioventricular; IV, indeterminate ventricle; LV, left ventricle; RV, right ventricle.
The VA connections were seen as concordant in six cases (i.e. Holmes heart), and discordant in 32. Double outlet LV was seen in one, and pulmonary atresia (i.e. single aortic outlet) was seen in two cases.
In eight cases, an imperforate pulmonary valve was noticed – five with discordant and three concordant VA connections. The associated anomalies are seen in Table 3.
Double inlet ventricle: associated anomalies.
| Associated anomalies | Double inlet LV: 49 cases | Double inlet RV: 28 cases | Double inlet IV: 2 cases | ||
|---|---|---|---|---|---|
| Dextrocardia | – | 4 | – | ||
| Persistent LSVC to coronary sinus | 1 | 2 | – | ||
| Interrupted IVC | – | 3 | – | ||
| TAPVR | 10 | SVC: 5 | 11 | SVC: 7 | – |
| Atria: 4 | Atria: 4 | ||||
| IVC: 1 | IVC: – | ||||
| ASD | 25 | 13 | 1 | ||
| PFO | 20 | 1 | – | ||
| Common atrium | 3 | 14 | – | ||
| Parachute MV | 1 | – | – | ||
| AV septal defect | 9 | 20 | – | ||
| VSD | 46 | 3 | – | ||
| Subaortic infundibulum | 33 | 8 | – | ||
| LVOTO | 8 | – | – | ||
| RVOTO | 2 | 2 | – | ||
| BAV | 4 | – | – | ||
| Right sided AoA | 2 | 4 | 1 | ||
| COA | 6 | – | – | ||
| Interrupted AoA | 2 | – | – | ||
| PDA | 40 | 20 | – | ||
Abbreviations: AOA, aortic arch; ASD, Atrial septal defect; AV, atrioventricular; BAV, bicuspid aortic valve; COA, coarctation of aorta; IV, indeterminate ventricle; IVC, inferior vena cava; LSVC, left superior vena cava; LV, left ventricle; LVOTO, left ventricular outflow obstruction; MV, mitral valve; PDA, patent ductus arteriosus; PFO, patent foramen ovale; RV, right ventricle; RVOTO, right ventricular outflow obstruction; SVC: superior vena cava; TAPVR, total anomalous pulmonary venous return; VSD, ventricular septal defect.
In these hearts, the RV is the dominant one with coarse apical trabecular morphology. The LV was hypoplastic. We noticed that two patent AV valves were seen in eight cases, common AV valve in 20 cases, and no cases with atretic right or left valve.
The types of the VA connections in DIRV are seen in Table 4.
Double-inlet indeterminate ventricle (DIIV): 2 casesTwo cases were seen with only one chamber identified with coarse trabeculation of indeterminate anatomy. Two patent AV valves and double outlet VA connection were noticed. Atrial septal defect (ASD) and right sided aortic arch were seen in one case (Table 3).
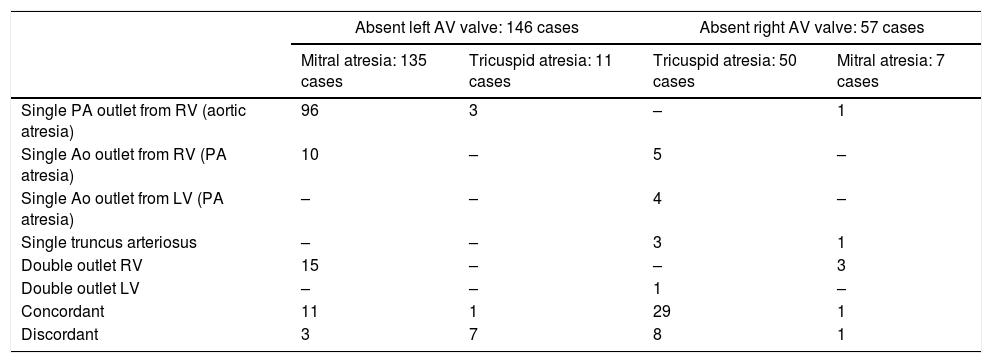
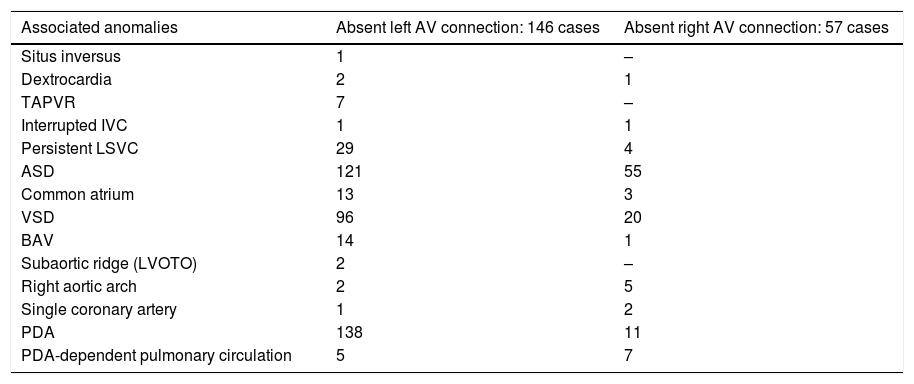
Absent AV connectionTwo-hundred and three cases with absent AV connections. They are divided into two groups: absent left connection (146 cases; 72%) and absent right connection (57 cases; 28%).
Absent left AV connection: 146 casesIn 135 cases (92%) the atretic valve was mitral and resulting in D-looping of the heart, and in the remaining 11 cases (8%), the tricuspid valve is atretic with resultant L-looping.
In cases of mitral atresia, the valve is imperforated in 14 cases (10%) and absent in the remaining 121 (90%). The tricuspid valve was seen straddling in 6 cases (5%) among those with absent mitral valve. Hypoplastic LV was seen in all cases.
A detailed description of the VA connections is seen in Table 5.
Absent AV connection: VA connection.
| Absent left AV valve: 146 cases | Absent right AV valve: 57 cases | |||
|---|---|---|---|---|
| Mitral atresia: 135 cases | Tricuspid atresia: 11 cases | Tricuspid atresia: 50 cases | Mitral atresia: 7 cases | |
| Single PA outlet from RV (aortic atresia) | 96 | 3 | – | 1 |
| Single Ao outlet from RV (PA atresia) | 10 | – | 5 | – |
| Single Ao outlet from LV (PA atresia) | – | – | 4 | – |
| Single truncus arteriosus | – | – | 3 | 1 |
| Double outlet RV | 15 | – | – | 3 |
| Double outlet LV | – | – | 1 | – |
| Concordant | 11 | 1 | 29 | 1 |
| Discordant | 3 | 7 | 8 | 1 |
Abbreviations: Ao, aorta; AV, atrioventricular; LV, left ventricle; PA, pulmonary artery; RV, right ventricle; VA, ventriculoarterial.
Associated anomalies are seen in Table 6.
Absent AV connection: associated anomalies.
| Associated anomalies | Absent left AV connection: 146 cases | Absent right AV connection: 57 cases |
|---|---|---|
| Situs inversus | 1 | – |
| Dextrocardia | 2 | 1 |
| TAPVR | 7 | – |
| Interrupted IVC | 1 | 1 |
| Persistent LSVC | 29 | 4 |
| ASD | 121 | 55 |
| Common atrium | 13 | 3 |
| VSD | 96 | 20 |
| BAV | 14 | 1 |
| Subaortic ridge (LVOTO) | 2 | – |
| Right aortic arch | 2 | 5 |
| Single coronary artery | 1 | 2 |
| PDA | 138 | 11 |
| PDA-dependent pulmonary circulation | 5 | 7 |
Abbreviations: ASD, atrial septal defect; BAV, bicuspid aortic valve; IVC, inferior vena cava; LSVC, left superior vena cava; LVOTO, left ventricular outflow tract obstruction; PDA, patent ductus arteriosus; TAPVR, total anomalous pulmonary venous return; VSD, ventricular septal defect.
In the 11 cases of tricuspid atresia, the left AV valve was absent in all cases. The VA connections are of different kinds (Table 5).
Associated anomalies are seen in Table 6.
Absent right AV connection: 57 casesAn absent right AV connection is present in 57 cases in our data. In 50 cases (88%), the ventricles had D-loop relation and in 7 cases (12%), L-loop is seen. In the latter, the tricuspid valve was patent and the RV is well-developed.
In cases of D-looping (i.e. tricuspid atresia), the right AV valve is absent in 48 cases (96%) and imperforate in only two (4%). The VA connection is concordant in 30 (53%) and discordant in nine (16%) patients. Other relations are seen in Table 5. In the nine cases of pulmonary atresia (whether the PA originates from the RV or LV), there is PDA supplying the pulmonary circulation in seven cases (78%); while in two cases (22%), the source is not identified. The associated anomalies are seen in Table 6.
In the seven cases, where the mitral valve is atretic and the relation is L-loop, the mitral valve is absent in all cases with straddling of the left AV valve in two cases (29%). The LV is hypoplastic and the dominant chamber is of RV morphology in all cases. The most common VA connection noticed is DORV (3 cases; 43%). However, other relations are seen as in Table 5.
Associated abnormalities are seen in Table 6.
DiscussionUniventricular heart (including the functional type) was identified in 1.4% of CHD cases in our study; results that are similar to those identified by other researchers elsewhere in the world.12,13
Debates and discussions in the past century have attempted to analyse and name these pathologies appropriately. Single ventricle, common ventricle, cor triloculare biatriatum, cor biloculare, and lately univentricular heart are examples of terms adopted to describe this entity.14
The normal ventricles – in the past – were termed to have a sinus (i.e. inlet) and conus (i.e. outlet) parts.15,16 Then after, they were regarded as having three components: inlet, trabecular, and outlet.17 In hearts with abnormal AV connection, this tripartite approach, in addition to the morphology of the interventricular septum, allows identifying left and right ventricles.
The first extensive review was published by Van Praagh et al.14 in 1965. They studied 60 necropsy hearts with single ventricle. They described those hearts as having univentricular anatomy. They used the term single or common ventricle to describe a ventricular chamber which either receives both mitral and tricuspid valve or a common AV valve. However, they excluded mitral and tricuspid atresias. They used – interchangeably – the terms univentricular, single and common hearts. Furthermore, they believed that it is inappropriate to use the adjective ‘single’ to a larger ventricle when a small one is also present.
Correspondingly, Thiene et al.4,18 highlighted the importance of precise identification of ventricular morphology. In addition, they showed that a lot of cases labelled as mitral or tricuspid atresia are in fact double-inlet ventricles with one AV valve atresia.
Recently, Cook and Anderson8,19 stressed the importance of a ‘segmental’ analysis needed for classifying the hearts with functionally univentricular AV connections. Further, they pointed out that the morphology of the apical trabeculations is essential for ventricular determination and overall topologic arrangement. Fine septal trabeculations define the LV; whereas, coarse ones feature the anatomic RV.
These characteristics are identifiable also in hearts with a deficient outlet or inlet parts. There is the only exception which is the solitary or indeterminate ventricle with no septal residua and the apical portion is uniformly coarse.20
A ventricular chamber which lacks one component was not regarded as a true ventricle for decades. Double-inlet chamber was labelled single ventricle despite having two ventricular cavities, one large and another small with no inlet portion. Mitral or tricuspid atresia had the same consideration.21
The heart that shows two ventricles – one large and one small, hypoplastic – is called with different names such as single or common ventricle,2,15,16,22–26 primitive ventricle,27–29 cor triloculare biatriatum,30–34 double-inlet ventricle,35–38 and univentricular heart.39–47
The anatomy and morphology of the dominant ventricle was the most important aspect to be analysed and described in all of the definitions. Then after, AV connection – in addition to the dominant ventricle morphology – was considered.
A new term has emerged which is a ‘univentricular AV connection’. It implies that both atria, directly or indirectly, drain predominantly to the only single ventricle. It does not preclude the presence of two ventricular chambers, even though one of them might be hypoplastic and diminutive.40,48,49
Nowadays, a new term has become popular and being used increasingly by many paediatric cardiologists and surgeons. This term is ‘functionally’ univentricular hearts.7–11 Cases with biventricular AV connection (i.e. the atria connect separately to their own ventricles) are included. In the latter hearts, either the left or right ventricle is too small to be able to sustain the systemic or pulmonary circulation, respectively.
This is of surgical importance because single-ventricle repair is the treatment of choice in those scenarios. Take pulmonary valve atresia and intact ventricular septum as an example. Despite the fact that the RV is tripartite and has all the three components, it has a small diminutive cavity – because of extensive wall hypertrophy – and is unable to sustain a standard two-ventricle surgical repair.50
However, the surgical treatment of a ‘functionally’ univentricular heart varies considerably. It depends largely on the anatomy and the potential function of the ventricles. In some cases, for example, one ventricle is greatly dysfunctional or hypoplastic to a degree that only single-ventricle repair should be considered; while in others, the small ventricle might be a good substrate for a one-and-half ventricle or even two-ventricle correction.
In double-inlet LV (DILV) with D-looping, the most common reported VA connection is the discordant one. The VSD in this case – also called as bulboventricular foramen – might be obstructive and small which accounts for subaortic stenosis in 50% of cases.19,51 In comparison to the dilated PA, the aorta is usually hypoplastic, and obstruction in the form of coarctation or atresia can be seen commonly.6 In rare occasions, DILV might show concordant VA connection – also known as Holmes Heart – or both great arteries originate from hypoplastic RV (DORV) or the dominant LV (DOLV).
When the dominant ventricular chamber is the RV with coarse trabeculations, double-inlet RV (DIRV) is said to be present. The hypoplastic ventricle is the LV which is situated in a posteroinferior location. According to the type of the looping, it is either on the left or, rarely, the right of the RV.
Different kinds of VA connections can be seen in DIRV. DORV is the most common type in many studies6 – including ours. However, concordant, single outlet or rarely discordant VA connections were found.
Isomerism has not been recognised in our cases. The reason for this could be multifactorial. Either the examining physician or echocardiographic was unaware of these abnormalities at that time or isomerism was not present indeed. Nevertheless, isomerism has been reported with DIRV in many case series studies.44–46
In many cases of right isomerism, the existence of anomalous pulmonary venous return, visceral heterotaxy, and a common atrium can complicate the patients’ clinical presentation.6
It is preferred by many – including us – to describe the position of the ventricle to the septum (i.e. D- or L-loop) and the sidedness of the absent connection (i.e. left or right), rather than just by the nomination of the abnormality as being mitral or tricuspid atresia.52–54 In this way, heart with absent left AV connection and D-loop, the atretic valve is mitral and the patent one is the tricuspid and so forth. Absent right AV connection coupled with D-looping is commonly associated with VA connection of concordant relation.52
In this scenario, the size of the RV, as well as the PA, depends largely on the size of the VSD. Likewise, when the VA connection is discordant and the VSD is small and restrictive, aortic hypoplasia or even atresia might be seen.55
Aortic atresia with the single pulmonary outlet is present in most cases of absent left AV connection.52 The LV is primitive and, surprisingly, the presence of VSD does not alter the clinical presentation.6
Critical aortic stenosis might be associated with hypoplasia and endocardial fibroelastosis of the LV. In this setting, a one-and-half repair is the only viable option, despite the fact that all components of the LV are present.6
A similar scenario is seen in cases of critical PS or atresia associated with hypoplastic, hypertrophied RV with evidence of endocardial fibroelastosis.50
On the other hand, when pulmonary or aortic atresia is associated with a large VSD, the growth of either ventricle can be enough to permit two-ventricle repair.
Extreme atrialization of the RV can be seen of Ebstein anomaly of the tricuspid valve. One portion of the RV cavity is said to be lost due to downward displacement of the posterior and septal leaflets, leaving a right ventricle with only outlet and apical portions.56 In this setting, one-ventricle or – in the best circumstances – a one-and-half surgical repair is only feasible.
ConclusionThe term univentricular heart is to be adopted instead of many confusing, ever-changing terms. However, detailed anatomical descriptions need to be pointed out which is relevant for the future-planning of the type of the surgical repair.
Furthermore, the definition of the functionally univentricular heart is increasingly recognised nowadays. It encompasses ventricles that possess all the morphological portions but where one of them is too weak to sustain the circulation when a biventricular surgical repair is considered.57–59 It is hoped that this long-term study will be the basis and a reference to paediatric cardiologists, echocardiographers, and other interested professionals.
FundingNil.
Conflict of interestNothing to declare.
The author acknowledges Professor Sadiq M. Al-Hamash for assisting in data collection, interpretation and analysis.