To investigate long term survival (15 years) and major morbidity in patients aged 50–65 years undergoing primary isolated aortic valve replacement with bioprosthetic or mechanical valves.
MethodsA single center retrospective analytical study of all patients aged 50–65 years with severe aortic stenosis who underwent surgery between 2000 and 2015 was performed (n=200). Two groups, mechanical (n=117) and biological (n=83) were obtained. Propensity score matching was performed for analysis. Primary outcome was survival, secondary outcome was major adverse cardiovascular complications (30-day mortality, stroke, any prosthesis-related reoperation and major bleeding).
ResultsMean age was 60±4 years, 33% female, mean follow up was 8.2±3 years (range 0–17 years). Matched overall survival was similar between groups, 65% at 15 years [Log Rank p=0.71, hazard ratio 0.87 (95% CI, 0.41–1.82)]. After matching, mechanical prosthesis presented a trend toward of more major adverse cardiovascular complications (30% versus 15%, p=0.07) with more major bleedings (15% versus 6.3%, p=0.06), stroke 11% versus 7.6% (p=0.44), and cardiac-related rehospitalization (33.7% versus 21.5%, p=0.06). Reoperation was nonsignificant between groups (2.5% mechanical versus 6.3% bioprosthesis, with only 2 cases of structural valve degeneration). Follow up mean transprosthetic gradients were higher in the mechanical group (18±6 versus 15±7mmHg, p=0.01).
ConclusionsAmong propensity matched patients there were no differences in survival between groups at 15 years. The mechanical prosthesis presented a trend toward twofold more major adverse cardiovascular complications specially due to major bleeding. Studies with larger sample sizes are needed to confirm these results.
Investigar la supervivencia a largo plazo (15 años) y la morbilidad en pacientes de 50 a 65 años sometidos a reemplazo valvular aórtico aislado con válvulas bioprotésicas o mecánicas.
MétodosEstudio analítico retrospectivo unicéntrico, de pacientes de 50-65 años con estenosis aórtica severa que se sometieron a cirugía entre 2000-2015 (n=200). Se obtuvieron 2 grupos, mecánico (n=117) y biológico (n=83). Se realiza puntuación de propensión para el análisis final. El objetivo primario fue la supervivencia a largo plazo y como objetivos secundarios las complicaciones cardiovasculares mayores (mortalidad a 30 días, ictus, reintervención protésica y hemorragia grave).
ResultadosLa edad media fue 60±4 años, un 33% mujeres. El seguimiento medio fue de 8,2±3 años. La supervivencia global fue similar, del 65% a los 15 años (log rank p=0,71, hazard ratio 0,87 [IC 95%: 0,41-1,82]). Después del pareado las prótesis mecánicas presentaron una tendencia hacia más complicaciones cardiovasculares mayores (30% vs 15%, p=0,07) con más hemorragias mayores (15% vs 6,3% p=0,06), ictus (11% vs 7,6%, p=0,44) y rehospitalización de causa cardíaca (33,7% vs 21,5%, p=0,06). La reintervención no fue significativa entre grupos (2,5% mecánicas vs 6,3% bioprótesis, 2 casos de degeneración valvular estructural). Los gradientes transprotésicos fueron mayores en el grupo mecánico (18±6 vs 15±7mmHg, p=0,01).
ConclusionesNo hubo diferencias en la supervivencia a 15 años. Las prótesis mecánicas presentaron una tendencia al doble de complicaciones mayores, especialmente debido a sangrado mayor. Se necesitan estudios con mayor tamaño muestral para confirmar estos datos.
The standard treatment for patients with severe aortic valve disease, aortic valve replacement (AVR), is performed in ∼280,000 patients worldwide every year,1 and ∼5000 in Spain.2 Recent 2017 guidelines from the European Society of Cardiology/European Association of Cardiothoracic Surgery (ESC/EACTS) maintain that a bioprosthesis should be considered in patients above 65 years of age.3 In patients aged between 60 and 65 years, both valve types are considered acceptable options (class IIa indication). According to the recent focused 2017 update American Heart Association/American College of Cardiology (AHA/ACC) guidelines,4 the choice of prosthetic heart valve should be a shared decision-making process according to patient desire. Below 70 years old, the age range was expanded from age 60–70 to age 50–70 for either a mechanical or bioprosthetic valve choice (class IIa indication).
Recently two great and large observational studies with patients between 50 and 69 years were published with contradictory results after a 15-year follow up. Chiang et al.5 in an American cohort in 2014, published that bioprosthesis could be considered for patients down to 50 years of age, which is supported by other studies.6 Glaser et al.,7 in a Swedish cohort concluded in 2016 that patients aged 50–69 years who received mechanical valves had better long-term survival. The risk of stroke was similar; however, patients with bioprosthesis had a higher risk of reoperation and a lower risk of major bleeding in both studies.
Nevertheless, biologic AVR in patients aged 50–65 years remains controversial,8 and the expected event rates for both perioperative and long-term valve-related complications have not been clearly determined in this patient subset in a mediterranean population. Our objective was to quantify long term survival (15 years) and major morbidity (rates of stroke, aortic valve reoperation, and major bleeding events) in a group of patients aged 50–65 years undergoing primary isolated aortic valve replacement with bioprosthetic or mechanical valves due to severe aortic stenosis between 2000 and 2015.
MethodsStudy designA single center retrospective analytical study of all patients aged 50–65 years with severe aortic stenosis who underwent primary isolated AVR surgery between 2000 and 2015 was performed. The study was approved by the ethics committee the 27th April 2017 (Comité de Ética de la Investigación Provincial de Málaga, Secretary Gloria Luque). All patients have given informed consent before participation in the study. Exclusion criteria were out-of-state residency, need for concomitant surgery, previous cardiac surgery and infective endocarditis. Two groups, mechanical (M, n=117) and biological (B, n=83) were obtained.
A crude analysis of the data and a posterior paired analysis by propensity score matching with IBM SPSS Statistics 22.0 for Windows (IBM Corp., Armonk, NY, USA) were carried out using a 1:1 “nearest neighbour” matching protocol based on the total number of bioprosthesis, creating a sample size of 166 patients, 83 per group for comparison.
Study endpointsThe primary outcome measure was overall survival. Secondary outcome was a combined endpoint of 4 major adverse cardiovascular events (MACCE): 30-day mortality, stroke, any prosthesis-related reoperation and major bleeding, according. to the Valve Academic Research Consortium 2 (VARC2) definitions.9
Preoperative characteristics, deaths and MACCE were identified using the Diraya Health Care medical records software (Servicio Andaluz de Salud, Spain) and the Cardiovascular Surgery Department local database, by searching all hospital admissions and ambulatory or emergency department visits for patient deaths or complications, and confirmed by direct telephone contact with the patient if alive and/or relatives if not.
Patients for whom no stroke, reoperation, or major bleeding event and no date of death were found were censored on December 31, 2016 (last follow-up date).
Echocardiographic data were recorded from same sources, including Cardiology Department local database, using the most recent echocardiogram.
Statistical methodAll analysis was performed with the IBM SPSS Statistics 22.0 for Windows software package. Continuous variables are reported as mean±SD. Categorical variables are expressed as absolute frequencies (n) and proportions (%). Baseline differences between patients receiving bioprosthetic or mechanical prosthetic valves were detected using t test for normally distributed continuous variables and Pearson χ2 test for categorical variables. In cases where normality cannot be accepted, the corresponding non-parametric test was applied. To adjust for differences in baseline characteristics and selection bias, propensity score matching was performed using by 1:1 nearest neighbor matching protocol without replacement, and a caliper width equal to 0.2 of the SD of the logit of the propensity score. All baseline characteristics (age, sex, logistic EuroScore, body mass index, hypertension, diabetes mellitus, atrial fibrillation, chronic obstructive pulmonary disease, dyslipidemia, previous stroke, previous myocardial infarction, chronic kidney disease, preoperative creatinine value, peripheral arteriopathy, transaortic mean gradients and left ventricular ejection fraction), were included as covariates in the propensity score model.
Kaplan Meier survival curves for the primary end point of survival were constructed for the entire study population as well as the propensity-matched groups. The difference in survival was assessed using the Log-Rank Mantel Cox test and 95% confidence interval hazard ratio was calculated using Cox proportional hazards regression. A sensibility Rosembaum test was performed satisfactorily.
Missing data were handled by estimating separate logistic regression models such as to maximize the number of included variables for each patient. A p-value ≤0.05 was considered statistically significant.
ResultsStudy populationWe identified all patients aged 50–65 years who met inclusion and exclusion criteria. A total of 215 patients were detected with primary isolated AVR but only 200 were included in the study (15 excluded because out-of state residency/endocarditis). Follow up was 98% completed. Of these, 58% (117/200) had received mechanical valves and 42% (83/200) bioprosthesis. The mean and maximum follow-up times were 7.8±4.5 and 17 years in the overall cohort, 9.7±4.3 and 17 years in the mechanical valve group, and, 6.1±3.1 and 17 years in the bioprosthetic valve group (p=0.001). The use of bioprosthesis increased from 13% all AVRs in 2000–2008 to 72% in 2009–2015, while the mean patient age per calendar year remained relatively constant around 60 years. Bioprosthesis were 80% Carpentier-Edwards Perimount (Edwards Lifesciences, Irvine, USA), 10% Mitroflow (Sorin Group, Saluggia, Italy), 7% Stentless Freedom Solo (Sorin Group, Saluggia, Italy) and 3% Stentless Porcine Toronto (St. Jude Medical, Inc., St. Paul, Minn). All mechanical valves were Carbomedics (Sorin Group, Saluggia, Italy).
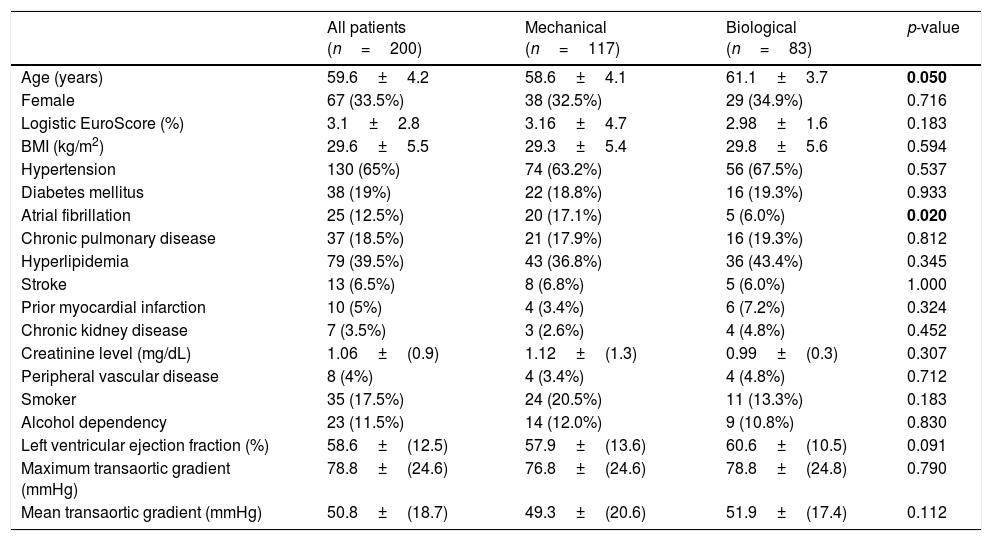
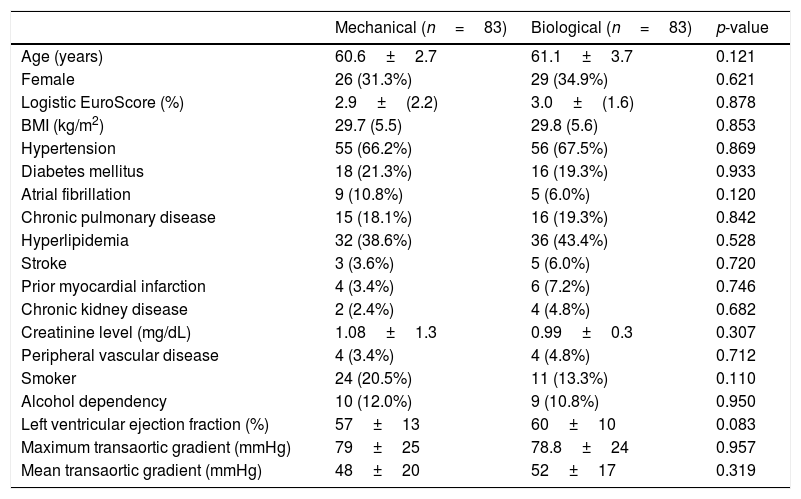
Patient characteristicsThe baseline patient characteristics in the overall cohort are shown in Table 1. The mean age was 58.6 years in patients who had received mechanical valves and 61.1 years in those who had received bioprosthetic valves (p=0.05). Compared with the patients who received bioprosthesis valves, the patients who had received mechanical valves were 2.5 years younger and had more preoperative chronic atrial fibrillation (17% versus 6%, p=0.02). In the propensity score-matched cohort (83 pairs), all baseline characteristics were well balanced, as shown in Table 2.
Patient baseline characteristics (overall unadjusted cohort).
| All patients (n=200) | Mechanical (n=117) | Biological (n=83) | p-value | |
|---|---|---|---|---|
| Age (years) | 59.6±4.2 | 58.6±4.1 | 61.1±3.7 | 0.050 |
| Female | 67 (33.5%) | 38 (32.5%) | 29 (34.9%) | 0.716 |
| Logistic EuroScore (%) | 3.1±2.8 | 3.16±4.7 | 2.98±1.6 | 0.183 |
| BMI (kg/m2) | 29.6±5.5 | 29.3±5.4 | 29.8±5.6 | 0.594 |
| Hypertension | 130 (65%) | 74 (63.2%) | 56 (67.5%) | 0.537 |
| Diabetes mellitus | 38 (19%) | 22 (18.8%) | 16 (19.3%) | 0.933 |
| Atrial fibrillation | 25 (12.5%) | 20 (17.1%) | 5 (6.0%) | 0.020 |
| Chronic pulmonary disease | 37 (18.5%) | 21 (17.9%) | 16 (19.3%) | 0.812 |
| Hyperlipidemia | 79 (39.5%) | 43 (36.8%) | 36 (43.4%) | 0.345 |
| Stroke | 13 (6.5%) | 8 (6.8%) | 5 (6.0%) | 1.000 |
| Prior myocardial infarction | 10 (5%) | 4 (3.4%) | 6 (7.2%) | 0.324 |
| Chronic kidney disease | 7 (3.5%) | 3 (2.6%) | 4 (4.8%) | 0.452 |
| Creatinine level (mg/dL) | 1.06±(0.9) | 1.12±(1.3) | 0.99±(0.3) | 0.307 |
| Peripheral vascular disease | 8 (4%) | 4 (3.4%) | 4 (4.8%) | 0.712 |
| Smoker | 35 (17.5%) | 24 (20.5%) | 11 (13.3%) | 0.183 |
| Alcohol dependency | 23 (11.5%) | 14 (12.0%) | 9 (10.8%) | 0.830 |
| Left ventricular ejection fraction (%) | 58.6±(12.5) | 57.9±(13.6) | 60.6±(10.5) | 0.091 |
| Maximum transaortic gradient (mmHg) | 78.8±(24.6) | 76.8±(24.6) | 78.8±(24.8) | 0.790 |
| Mean transaortic gradient (mmHg) | 50.8±(18.7) | 49.3±(20.6) | 51.9±(17.4) | 0.112 |
Categorical data are expresses as n (%). Continuous variables are reported as mean±SD. BMI: body mass index.
Bold letters: statistically significant values.
Patient baseline characteristics after matching.
| Mechanical (n=83) | Biological (n=83) | p-value | |
|---|---|---|---|
| Age (years) | 60.6±2.7 | 61.1±3.7 | 0.121 |
| Female | 26 (31.3%) | 29 (34.9%) | 0.621 |
| Logistic EuroScore (%) | 2.9±(2.2) | 3.0±(1.6) | 0.878 |
| BMI (kg/m2) | 29.7 (5.5) | 29.8 (5.6) | 0.853 |
| Hypertension | 55 (66.2%) | 56 (67.5%) | 0.869 |
| Diabetes mellitus | 18 (21.3%) | 16 (19.3%) | 0.933 |
| Atrial fibrillation | 9 (10.8%) | 5 (6.0%) | 0.120 |
| Chronic pulmonary disease | 15 (18.1%) | 16 (19.3%) | 0.842 |
| Hyperlipidemia | 32 (38.6%) | 36 (43.4%) | 0.528 |
| Stroke | 3 (3.6%) | 5 (6.0%) | 0.720 |
| Prior myocardial infarction | 4 (3.4%) | 6 (7.2%) | 0.746 |
| Chronic kidney disease | 2 (2.4%) | 4 (4.8%) | 0.682 |
| Creatinine level (mg/dL) | 1.08±1.3 | 0.99±0.3 | 0.307 |
| Peripheral vascular disease | 4 (3.4%) | 4 (4.8%) | 0.712 |
| Smoker | 24 (20.5%) | 11 (13.3%) | 0.110 |
| Alcohol dependency | 10 (12.0%) | 9 (10.8%) | 0.950 |
| Left ventricular ejection fraction (%) | 57±13 | 60±10 | 0.083 |
| Maximum transaortic gradient (mmHg) | 79±25 | 78.8±24 | 0.957 |
| Mean transaortic gradient (mmHg) | 48±20 | 52±17 | 0.319 |
Categorical data are expresses as n (%). Continuous variables are reported as mean±SD. BMI: body mass index.
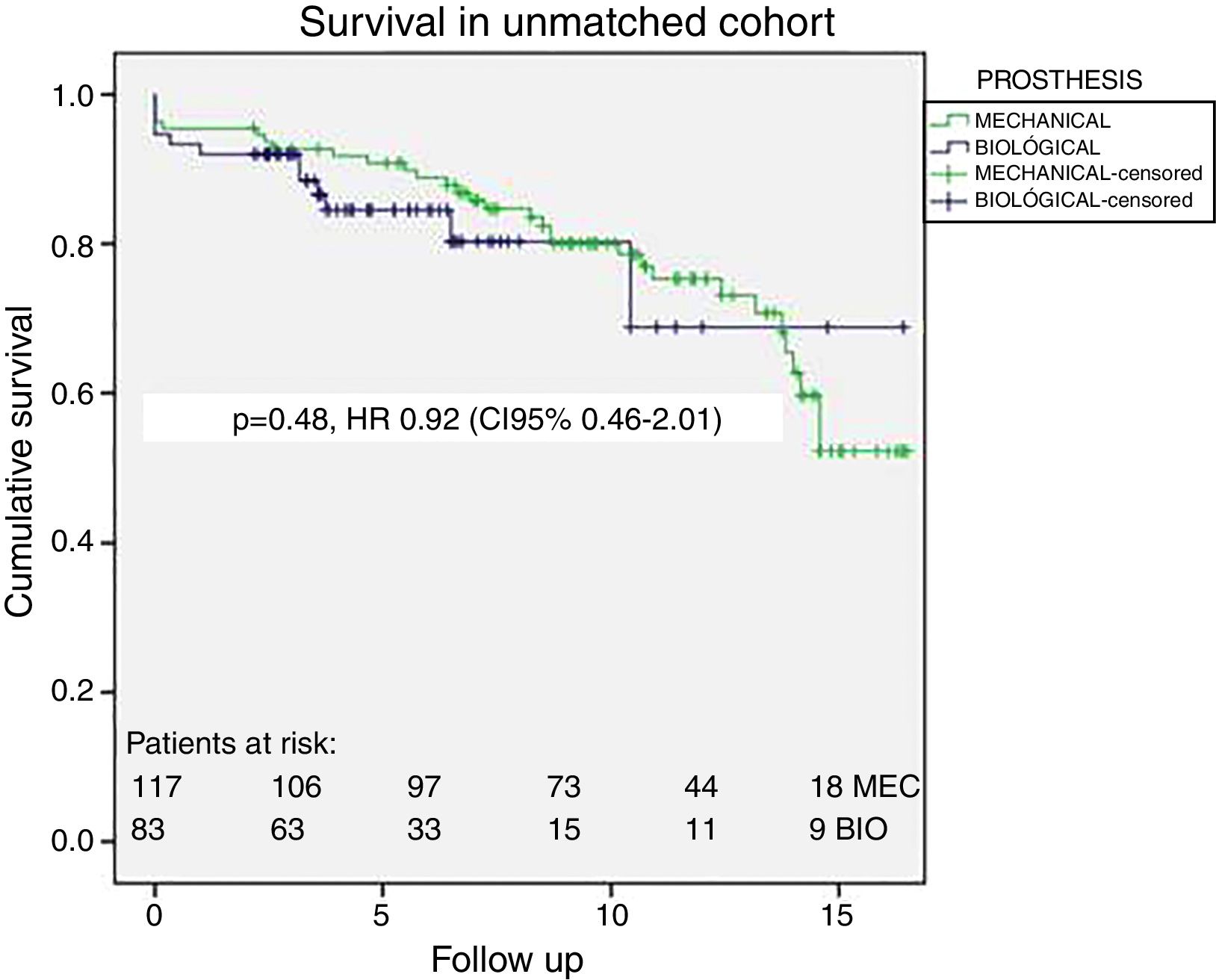
No differences in long-term survival were observed (log-Rank Mantel Cox p=0.71) with 90%, 80% and 65% survival at 5, 10 and 15 years respectively. Fig. 1 displays the survival curves for overall survival in the unmatched patients; propensity-matched cohort survival curves are shown in Fig. 2. In matched cohort there were 17 (20%) deaths in the bioprosthesis group and 21 (25%) deaths in the mechanical prosthesis group during a maximum of 17 years of follow-up. The hazard ratio for death for mechanical prosthesis versus bioprosthesis groups was 0.87 (95% CI, 0.41–1.82, p=0.710). No differences were found also in a subgroup analysis between patients aged 50–58 years (n=57, 21 B versus 36 M, Log-Rank Mantel Cox p=0.634) with 85% survival at 15 years in both groups.
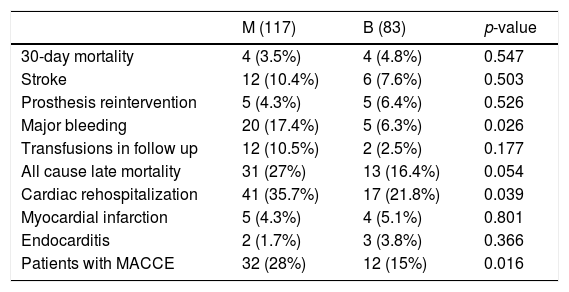
All MACCE complications in overall unmatched cohort are summarized in Table 3. The MACCE combined endpoint of the 4 major complications (30-day mortality, stroke, any prosthesis-related reoperation and major bleeding) was statistically significant favoring bioprosthesis (p=0.016). A total of 41 MACCE were detected in 32 patients in M group and 20 MACCE in 12 patients in B group, mainly due to major bleeding (p=0.02). There were no differences in the rest of complications, except higher rates of cardiac readmission in M group (p=0.039).
Major adverse cardiovascular events (MACCE) in unmatched overall cohort.
| M (117) | B (83) | p-value | |
|---|---|---|---|
| 30-day mortality | 4 (3.5%) | 4 (4.8%) | 0.547 |
| Stroke | 12 (10.4%) | 6 (7.6%) | 0.503 |
| Prosthesis reintervention | 5 (4.3%) | 5 (6.4%) | 0.526 |
| Major bleeding | 20 (17.4%) | 5 (6.3%) | 0.026 |
| Transfusions in follow up | 12 (10.5%) | 2 (2.5%) | 0.177 |
| All cause late mortality | 31 (27%) | 13 (16.4%) | 0.054 |
| Cardiac rehospitalization | 41 (35.7%) | 17 (21.8%) | 0.039 |
| Myocardial infarction | 5 (4.3%) | 4 (5.1%) | 0.801 |
| Endocarditis | 2 (1.7%) | 3 (3.8%) | 0.366 |
| Patients with MACCE | 32 (28%) | 12 (15%) | 0.016 |
Categorical data are expresses as n (%). Transfusions mean red blood packed cell transfusions due to anemia without an evident bleeding.
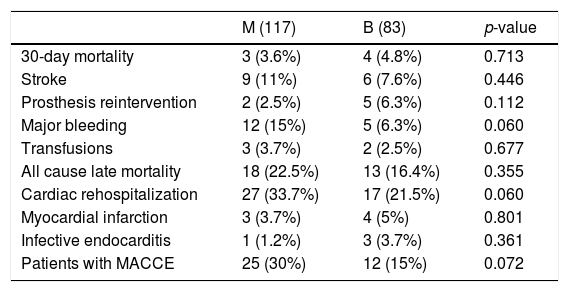
After matching (Table 4), there were 26 MACCE in 25 patients in M group and 20 MACCE in 12 patients in B group, without reaching the statistical significance but with a trend toward twofold MACCE in the Mechanical group (30% versus 15%, p=0.07), mainly due to major bleeding (p=0.06), with more cardiac readmissions in M group (p=0.06).
Major adverse cardiovascular events (MACCE) in matched cohort.
| M (117) | B (83) | p-value | |
|---|---|---|---|
| 30-day mortality | 3 (3.6%) | 4 (4.8%) | 0.713 |
| Stroke | 9 (11%) | 6 (7.6%) | 0.446 |
| Prosthesis reintervention | 2 (2.5%) | 5 (6.3%) | 0.112 |
| Major bleeding | 12 (15%) | 5 (6.3%) | 0.060 |
| Transfusions | 3 (3.7%) | 2 (2.5%) | 0.677 |
| All cause late mortality | 18 (22.5%) | 13 (16.4%) | 0.355 |
| Cardiac rehospitalization | 27 (33.7%) | 17 (21.5%) | 0.060 |
| Myocardial infarction | 3 (3.7%) | 4 (5%) | 0.801 |
| Infective endocarditis | 1 (1.2%) | 3 (3.7%) | 0.361 |
| Patients with MACCE | 25 (30%) | 12 (15%) | 0.072 |
Categorical data are expresses as n (%). Transfusions mean red blood packed cell transfusions due to anemia without an evident bleeding.
Prosthesis related reoperation were similar between unadjusted and matched groups, with only 2 cases of structural valve deterioration (SVD), reoperated on in year 6 and 14 after surgery in the bioprosthesis group, versus 1 case of pannus and 2 of thrombosis that also required reoperation in the mechanical one. There were similar rates of infective endocarditis, and in both cohorts mean transprosthetic aortic gradients were higher in mechanical group at follow up (18.3±6 versus 15.5±7mmHg, p=0.01), despite similar implanted prosthetic sizes (22±2mm in M versus 22.3±2mm in B, p=0.09).
DiscussionCurrent choice of prosthetic heart valve type should be a shared decision-making according to patient desire, after an exhaustive information. 2017 EACTS/ESC valvular guidelines state that a bioprosthetic or mechanical prosthetic aortic valve is reasonable in patients aged 60–65 years, being a mechanical valve replacement reasonable in patients younger than 60 years.3 In the previous 2017 update on AHA/ACC guidelines state the age range among patients undergoing AVR, was expanded to age 50–70 for either a mechanical or bioprosthetic valve choice.4
These age cutoffs are set at the point where the benefit of bioprosthesis (no anticoagulation) outweighs the risk of reoperation because of SVD, because younger age at implantation is directly associated with SVD.3,4,8 This change in the cutoff value is supported because current bioprosthesis appear to have lower rates of SVD due to new anticalcification treatments than those used during the previous randomized controlled trials (RCT),10–12 involving first-generation bioprosthesis, so the findings of these studies may no longer reflect outcomes in current clinical practice. Also, because the risks of reoperation have continued to decrease over time, being young patients undergoing AVR more often reluctant to accept the activity constraints associated with anticoagulants.
Prior non-randomized studies investigating survival and clinical outcomes following AVR with mechanical or bioprosthetic valves in middle-aged patients have reported contradictory results. Some studies have reported better long-term clinical outcomes in patients who received mechanical valves,7,13,14 whereas others have reported no significant difference in long-term survival between middle-aged patients who received bioprosthesis or mechanical valves, stating that the type of prosthetic valve does not influence overall mortality.5,6,15
Also in a large meta-analysis of 32 articles with follow-up over 10 years with 17,439 patients and 101,819 patient-years receiving AVR, they did not find significant differences in mortality between mechanical and biological valves in all age groups.16 Even in the age group below age 60, no survival difference in either AVR or MVR was reported after 20-year of follow-up.
The two most recent and larger (over 1000 propensity score matched patients per group) observational studies (Chiang et al.,5 and Glaser et al.,7 in patients aged 50–69 years still presented contradictory results. The former in 2014 found no differences in survival (HR 0.97, 95% CI, 0.83–1.14, p=0.74), but higher bleeding rates in mechanical valves (12% versus 7%, p=0.001) and higher reoperation ones in biological valves (12% versus 6%, p=0.001) with the same stroke rates (8%, p=0.84) at 15 years. The latter in 2016 found higher survival in the mechanical group (HR 1.34, 95% CI, 1.09–1.66, p=0.006) with similar MACCE results than Chiang et al.: higher bleeding rates in the mechanical group (16% versus 8%, p=0.001) and more reoperations in the biological one (8% versus 4% p=0.001), with the same stroke rates (12%, p=0.84) at 15 years. This difference could be explained due to the excellent quality of anticoagulation control performed in Sweden, which has repeatedly been shown to be very good.17 Thus, this high quality of anticoagulation management may have favorably affected clinical outcomes in patients with mechanical heart valves.
After carefully reading these studies, we decided to perform our study in a Mediterranean population, were body surface areas and valve sizes tend to be smaller than in other countries.18 The average patient was a male about 60 years old and overweighted. The analysis of our data suggest a trend toward the double of MACCE complications in mechanical versus biological groups, with similar survivals at 15 years, although not reaching statistically significant differences, probably due to small sample size. Our results in MACCE are quite consistent with recent larger studies, with stroke rates (7% versus 11%, p=0.44) and major bleeding (15% versus 6%, p=0.06) favoring bioprosthesis and reoperation favoring mechanical valves (2.5% versus 6%, p=0.12), and with very low rates of SVD, only 2 patients needing reoperation (2.5%). In our bioprosthesis cohort, Carpentier-Edwards Perimount (Edwards Lifesciences, Irvine, CA) was used in 80% of patients, 15% were Mitroflow (Sorin group, Saluggia, Italy) and 5% other valves. Long term survival could even improve because of the increase in the use of a ministernotomy approach,19 that could also facilitate a reoperation if needed.20
In terms of current bioprosthesis durability, recently Bourguignon et al.,8 reported that AVR reduces life expectancy comparing with the 50–65 years old French general population. Above 50 years, the expected Carpentier-Edwards valve durability estimate was equal to or higher than life expectancy after AVR in 85% of patients, with a median expected valve durability of 19 years.21 Other prosthesis have shown excellent long term results as well.22,23 Despite reported concerns about the Mitroflow valve durability before the newest anticalcification treatment,24 recent studies do not demonstrate that the type of bioprosthesis choice influence long-term survival, with reported freedom from SVD at 10 years up to 96%.25
Even if a reoperation is required, reoperative AVR can be performed safely. Current outcomes have improved, with mortality rates around 5% in reoperative AVR26 or even 2.5% if minimally invasive procedures were used.20
In addition, emergence of transcatheter valve-in-valve (ViV) replacement appears to be an alternative to reoperation. This option is changing the dynamics of the discussion of the trade-offs between mechanical and bioprosthetic valves, although extensive long-term follow-up of transcatheter valves is not yet available, and not all bioprosthesis are suitable for a future ViV procedure, which will always require insertion of a smaller valve than the original one, and patient–prosthesis mismatch could be a potential problem, so it can be considered actually as an alternative only for high reoperative risk patients.27
The recent introduction of oral direct thrombin inhibitors and factor Xa inhibitor for the treatment of atrial fibrillation (AF) as an alternative to warfarin needs to be mentioned. Currently, these agents are not approved for mechanical valves28 but patients who have received bioprosthesis and in AF may have a choice between warfarin and new oral anticoagulants that are easier to manage with no dietary restriction and no repeated blood test. Therefore, the myth associated with the need for mechanical valve for patients already on anticoagulation therapy needs to be reconsidered.
Mechanical prosthesis were associated with a lower reoperation rate, but this was at the expense of more major bleeding events, experienced by 6% versus 15% at 15 years in our work, which is consistent with previous studies. The 30-day mortality after a major bleeding event in these studies was 13.2% so it is an important issue.5 Recent trial comparing low-intensity, self-monitored anticoagulation regimens with standard anticoagulation in patients with mechanical valves have not shown a significant reduction in major bleeding nor thromboembolic events.29 These outcomes, together with the well-recognized patient dissatisfaction with the prospect of a lifetime of anticoagulation, may partially explain the increasing use of bioprosthesis that we observed during the last decade.1,2
We believe that the evolving technology (new prosthesis designed for a future ViV), newer anticalcification treatments30 and TAVI evolution will promote a decrease in the range of age for bioprosthesis implantation rates in the next years.
This study has promoted “The Andalousian Aortic Valve Multicentric Study” involving 7 major cardiac surgery Centers (ANDALVALVE, ClinicalTrials.gov ID: NCT03239509), expecting above 1000 patients to confirm these data.
Limitations of the studyBecause this was an observational retrospective study, the issue of selection bias was addressed primarily by matching, using propensity score. However, it is only possible to match or adjust for patient characteristics that are known and have been measured and recorded. There may be unmeasured or unknown factors or both that we were unable to control for (residual confounding). This potentially could introduce a bias in favor of mechanical valves because surgeons tend to implant bioprosthetic valves in patients that they consider to have reduced life expectancy based on these or other factors. Another limitation was missing baseline data which potentially could have influenced our results.
The relatively small sample size has influenced the non-achievement of statistically significant values, despite the tendency to present fewer complications in the bioprosthesis group. According to sample size calculators, it would take 120 patients per group to obtain a statistical significance with a power of 80% and alpha 0.05.
The follow up echocardiographic data were not obtained at the same time point during the follow up. Interpersonal interpretations of echochardiograms could also underestimate the real SVD rate and gradients.
ConclusionsCurrently there is an increasing usage of bioprosthesis both at our institution and worldwide. Among propensity matched patients there were no differences in survival at 15 years, between mechanical and biological valves. The mechanical prosthesis presented a trend toward twofold more MACCE complications in matched sample specially due to major bleeding. Studies with larger sample sizes would be needed to confirm these data and so advice a decrease in the age for a bioprosthesis implantation.
Conflict of interestAll authors declare no conflict of interest.
Thanks to Medical student Francisco Martín for his support recording data.
This paper has been presented at the 31st European Association of Cardiothoracic Surgery (EACTS) Annual Meeting (7–11 October 2017) in Vienna, Austria as oral communication.