Given the scarcity of donors in pancreas transplantation,1 it is necessary to use expanded criteria to increase the graft pool. Controlled donation after cardiac death (DCD) (Maastricht III) is an additional effective source of organs that is widely recognized in kidney and liver transplantation, but there has been less experience in pancreas transplantation.2,3
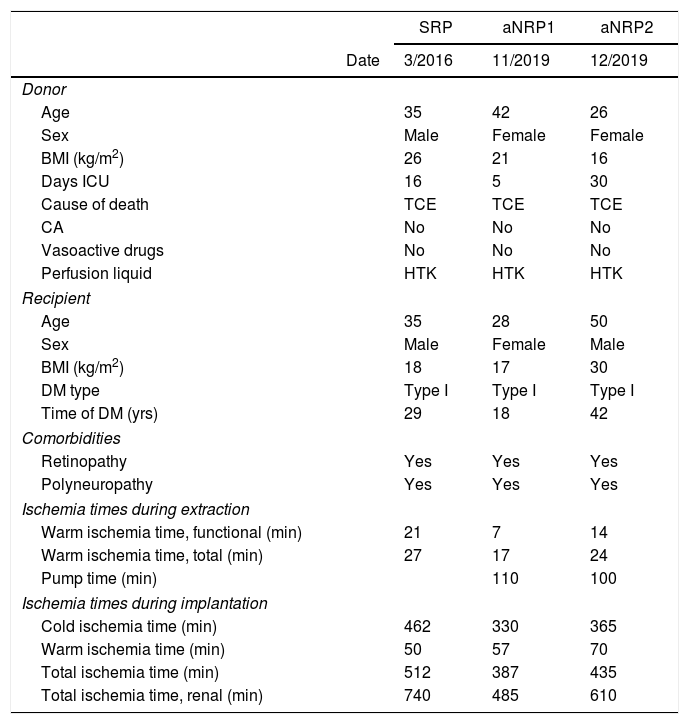
We present three cases of kidney–pancreas transplantation performed at our hospital with DCD: one using super-rapid procurement (SRP), and two cases of abdominal normothermic regional perfusion (aNRP). Table 1 shows the demographic characteristics of donors/recipients and ischemia times.
Donor/recipient characteristics and ischemia times.
| SRP | aNRP1 | aNRP2 | ||
|---|---|---|---|---|
| Date | 3/2016 | 11/2019 | 12/2019 | |
| Donor | ||||
| Age | 35 | 42 | 26 | |
| Sex | Male | Female | Female | |
| BMI (kg/m2) | 26 | 21 | 16 | |
| Days ICU | 16 | 5 | 30 | |
| Cause of death | TCE | TCE | TCE | |
| CA | No | No | No | |
| Vasoactive drugs | No | No | No | |
| Perfusion liquid | HTK | HTK | HTK | |
| Recipient | ||||
| Age | 35 | 28 | 50 | |
| Sex | Male | Female | Male | |
| BMI (kg/m2) | 18 | 17 | 30 | |
| DM type | Type I | Type I | Type I | |
| Time of DM (yrs) | 29 | 18 | 42 | |
| Comorbidities | ||||
| Retinopathy | Yes | Yes | Yes | |
| Polyneuropathy | Yes | Yes | Yes | |
| Ischemia times during extraction | ||||
| Warm ischemia time, functional (min) | 21 | 7 | 14 | |
| Warm ischemia time, total (min) | 27 | 17 | 24 | |
| Pump time (min) | 110 | 100 | ||
| Ischemia times during implantation | ||||
| Cold ischemia time (min) | 462 | 330 | 365 | |
| Warm ischemia time (min) | 50 | 57 | 70 | |
| Total ischemia time (min) | 512 | 387 | 435 | |
| Total ischemia time, renal (min) | 740 | 485 | 610 | |
SRP: super-rapid procurement; aNRP: abdominal normothermic regional perfusion; CA: cardiac arrest. Donor. Functional warm ischemia time: from the first significant hypotension (SBP<60mmHg) until preservation or start on pump. Total warm ischemia time: from the limitation life-support therapy (LLST) until preservation or on pump. Pump time: duration of aNRP. Recipient. Cold ischemia time: from preservation of the graft until its insertion in the surgical field. Warm ischemia time: from insertion of graft in the surgical field until perfusion. Total ischemia time: from graft preservation in the donor until perfusion in the recipient. Total ischemia time: from graft preservation in the donor until perfusion in the recipient.
Percutaneous premortem cannulation (cannulae inserted in the right femoral vessels, and balloon catheter in the supraceliac aorta at the left femoral level) was done in the ICU by the intensivist, and 1000U/kg of sodium heparin were administered. The intraoperative parameters of the aNRP met the recommendations of the Spanish National Transplant Organization.4
Super-rapid organ procurementUsing xipho-pubic laparotomy, the infrarenal aorta was cannulated and the supraceliac aorta was subsequently clamped, with immediate initiation of cold perfusion. The infrarenal vena cava was then cannulated for venous drainage, followed by portal cannulation and perfusion.
Surgical technique in the recipientPancreatic graft: arterial anastomosis between the donor iliac and the recipient right common iliac; venous anastomosis between the donor portal and the recipient right common cava-iliac; anastomosis between the donor duodenum and the recipient jejunal loop. The procedure was completed with standard appendectomy.
Renal graft: arterial anastomosis between the left external iliac artery and the renal artery aortic patch; venous anastomosis between the left external iliac vein and the renal; ureterostomy with a double J catheter stent.
Anticoagulation protocolThe anticoagulation protocol included: enoxaparin 40mg within 6h prior to transplantation, heparin sodium 2500 units intravenously (IV) administered intraoperatively in high-risk cases (elderly recipients, severe atherosclerosis, or long-term hemodialysis), and enoxaparin 60mg/24h 12hours after transplantation.
Immunosuppression protocolInduction: methylprednisolone (MP) 500mg IV, tacrolimus 0.1mg/kg, and mycophenolate mofetil (MMF) 1g oral. Intraoperative: thymoglobulin (Tg) 1.5mg/kg IV.
Maintenance: days 1–2 post-op: MP from 125mg/8h IV in a tapering regimen; day 3: prednisone 30mg/day oral. Tacrolimus: 0.1mg/kg/24h oral (levels 8–12ng/mL). Tg: 1.5mg/kg/day for five days. MMF 500mg/12h oral until day 5 post-op, then 1g/12h during the first month.
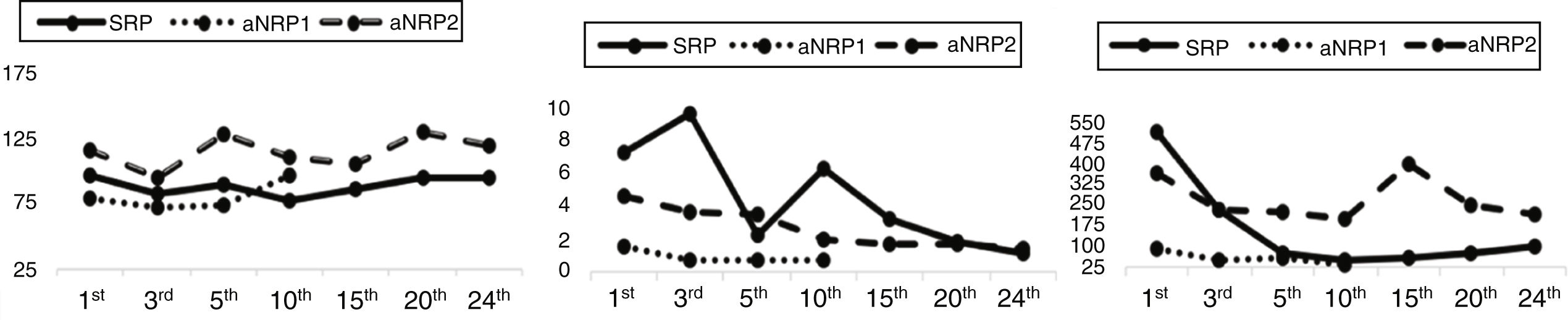
Evolution1strecipient: After reperfusion of the graft, the patient did not require insulin. Amylase levels normalized within 48h, and Doppler ultrasound follow-up studies were always adequate. After 60h, the patient presented hemoperitoneum and was reoperated without finding a clear source of hemorrhage. The kidney graft showed delayed function, requiring hemofiltration (HF) until the tenth day. The patient was discharged 24 days after surgery with normal blood glucose levels and no need for insulin, while creatinine levels were within normal ranges (Fig. 1).
2ndrecipient: The patient progressed with good function of both grafts. Initially, she required insulin infusion in the first 3h in the ICU (2u/mL), which was withdrawn after glucose levels normalized. Kidney function progressively improved without delay or need for HF. Patient was discharged on day 10 with normal parameters (Fig. 1) and no need for insulin.
Currently, these two recipients present correct function of both grafts.
3rdrecipient: Presented hemodynamic instability secondary to cardiogenic shock due to autonomic denervation syndrome, with continuous need for vasoactive drugs. Prolonged mechanical ventilation (MV) was needed secondary to pneumonia and subsequent acute respiratory distress syndrome. The kidney graft suffered secondary dysfunction with acute tubular necrosis (ATN) due to the hemodynamic situation, requiring HF throughout hospitalization. Blood glucose levels were initially adequate, although the patient required insulin infusion from the 15th day post-op until the end of hospital stay. On day 25, he developed septic shock secondary to a peripancreatic collection, which was treated with percutaneous drainage and antibiotics; pancreatic fistula was confirmed. The patient's condition worsened, requiring surgery for drainage and confirming the viability of the graft. He persisted in a hemodynamically labile situation and died 90 days after the initial surgery due to multiple organ failure.
We believe that the poor progress of this case is related to inadequate patient selection (50 years old, active smoker, BMI 30kg/m2, severe arteriosclerosis) rather than with the non-heart-beating donor or the procurement method.
The scarcity of pancreatic grafts from cadaveric donors makes DCD a new source of organs for transplantation.
The scarcity of pancreatic grafts from cadaveric donors makes DCD a new source of organs for pancreatic transplantation. The series by Kopp et al.5 with the super-rapid technique shows similar results to those of cadaveric donors following strict donor selection (age<50, BMI<30kg/m2, warm ischemia time<30min), as in our first case. In the literature, the studies by Oniscu et al.7 (three pancreas obtained by aNRP with post-mortem cannulation, although only one was used for kidney-pancreas transplantation) and Miñambres et al.8 (one case of kidney-pancreas transplantation) have reported results similar to those obtained with donation after brain death.
DCD appears to be a reliable source of organs with promising results in pancreatic transplantation. Both the super-rapid technique for procurement5,7,9 and the use of aNRP6–8 have shown encouraging results that are comparable to cadaveric donors. Future studies will help determine which technique may offer better results.
Please cite this article as: Gutiérrez Delgado MP, Sánchez Pérez B, Pérez Daga JA, León Díaz FJ, Santoyo Santoyo J. Donación en asistolia: un presente en el trasplante pancreático. Cir Esp. 2021;99:236–238.