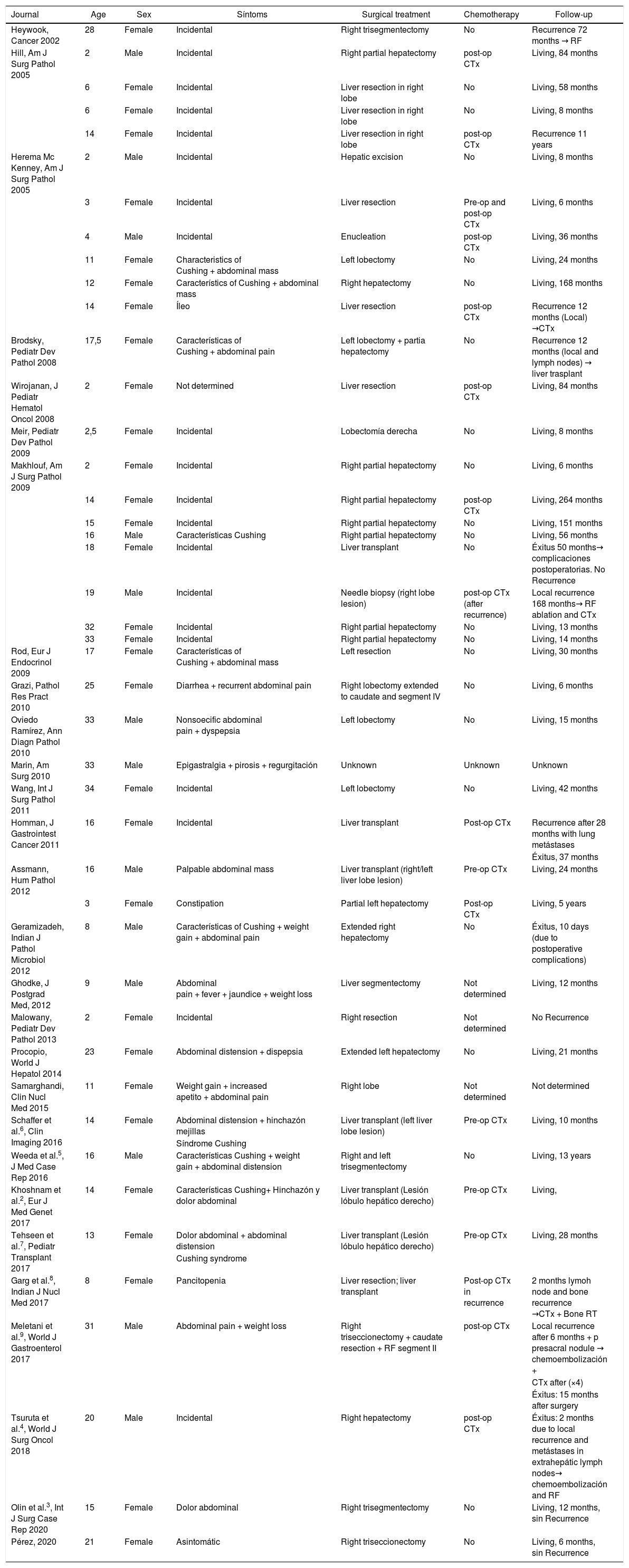
Calcifying nested stromal-epithelial tumors (CNSET) are rare primary liver tumors1–6 that were first described by Ishak in 20011,3,4,6. Only 43 cases have been published in the literature2–9 (Table 1). These neoplasms present an apparently benign and indolent clinical course, attributable to their low malignant potential10. Surgical treatment with free margins usually provides high long-term survival rates.
Clinical and pathlogical characterístics of 44 cases of calcifying nested stromal-epithelial tumor.
| Journal | Age | Sex | Síntoms | Surgical treatment | Chemotherapy | Follow-up |
|---|---|---|---|---|---|---|
| Heywook, Cancer 2002 | 28 | Female | Incidental | Right trisegmentectomy | No | Recurrence 72 months → RF |
| Hill, Am J Surg Pathol 2005 | 2 | Male | Incidental | Right partial hepatectomy | post-op CTx | Living, 84 months |
| 6 | Female | Incidental | Liver resection in right lobe | No | Living, 58 months | |
| 6 | Female | Incidental | Liver resection in right lobe | No | Living, 8 months | |
| 14 | Female | Incidental | Liver resection in right lobe | post-op CTx | Recurrence 11 years | |
| Herema Mc Kenney, Am J Surg Pathol 2005 | 2 | Male | Incidental | Hepatic excision | No | Living, 8 months |
| 3 | Female | Incidental | Liver resection | Pre-op and post-op CTx | Living, 6 months | |
| 4 | Male | Incidental | Enucleation | post-op CTx | Living, 36 months | |
| 11 | Female | Characteristics of Cushing + abdominal mass | Left lobectomy | No | Living, 24 months | |
| 12 | Female | Característics of Cushing + abdominal mass | Right hepatectomy | No | Living, 168 months | |
| 14 | Female | Íleo | Liver resection | post-op CTx | Recurrence 12 months (Local) →CTx | |
| Brodsky, Pediatr Dev Pathol 2008 | 17,5 | Female | Características of Cushing + abdominal pain | Left lobectomy + partia hepatectomy | No | Recurrence 12 months (local and lymph nodes) → liver trasplant |
| Wirojanan, J Pediatr Hematol Oncol 2008 | 2 | Female | Not determined | Liver resection | post-op CTx | Living, 84 months |
| Meir, Pediatr Dev Pathol 2009 | 2,5 | Female | Incidental | Lobectomía derecha | No | Living, 8 months |
| Makhlouf, Am J Surg Pathol 2009 | 2 | Female | Incidental | Right partial hepatectomy | No | Living, 6 months |
| 14 | Female | Incidental | Right partial hepatectomy | post-op CTx | Living, 264 months | |
| 15 | Female | Incidental | Right partial hepatectomy | No | Living, 151 months | |
| 16 | Male | Características Cushing | Right partial hepatectomy | No | Living, 56 months | |
| 18 | Female | Incidental | Liver transplant | No | Éxitus 50 months→ complicaciones postoperatorias. No Recurrence | |
| 19 | Male | Incidental | Needle biopsy (right lobe lesion) | post-op CTx (after recurrence) | Local recurrence 168 months→ RF ablation and CTx | |
| 32 | Female | Incidental | Right partial hepatectomy | No | Living, 13 months | |
| 33 | Female | Incidental | Right partial hepatectomy | No | Living, 14 months | |
| Rod, Eur J Endocrinol 2009 | 17 | Female | Características of Cushing + abdominal mass | Left resection | No | Living, 30 months |
| Grazi, Pathol Res Pract 2010 | 25 | Female | Diarrhea + recurrent abdominal pain | Right lobectomy extended to caudate and segment IV | No | Living, 6 months |
| Oviedo Ramírez, Ann Diagn Pathol 2010 | 33 | Male | Nonsoecific abdominal pain + dyspepsia | Left lobectomy | No | Living, 15 months |
| Marin, Am Surg 2010 | 33 | Male | Epigastralgia + pirosis + regurgitación | Unknown | Unknown | Unknown |
| Wang, Int J Surg Pathol 2011 | 34 | Female | Incidental | Left lobectomy | No | Living, 42 months |
| Homman, J Gastrointest Cancer 2011 | 16 | Female | Incidental | Liver transplant | Post-op CTx | Recurrence after 28 months with lung metástases |
| Éxitus, 37 months | ||||||
| Assmann, Hum Pathol 2012 | 16 | Male | Palpable abdominal mass | Liver transplant (right/left liver lobe lesion) | Pre-op CTx | Living, 24 months |
| 3 | Female | Constipation | Partial left hepatectomy | Post-op CTx | Living, 5 years | |
| Geramizadeh, Indian J Pathol Microbiol 2012 | 8 | Male | Características of Cushing + weight gain + abdominal pain | Extended right hepatectomy | No | Éxitus, 10 days (due to postoperative complications) |
| Ghodke, J Postgrad Med, 2012 | 9 | Male | Abdominal pain + fever + jaundice + weight loss | Liver segmentectomy | Not determined | Living, 12 months |
| Malowany, Pediatr Dev Pathol 2013 | 2 | Female | Incidental | Right resection | Not determined | No Recurrence |
| Procopio, World J Hepatol 2014 | 23 | Female | Abdominal distension + dispepsia | Extended left hepatectomy | No | Living, 21 months |
| Samarghandi, Clin Nucl Med 2015 | 11 | Female | Weight gain + increased apetito + abdominal pain | Right lobe | Not determined | Not determined |
| Schaffer et al.6, Clin Imaging 2016 | 14 | Female | Abdominal distension + hinchazón mejillas | Liver transplant (left liver lobe lesion) | Pre-op CTx | Living, 10 months |
| Síndrome Cushing | ||||||
| Weeda et al.5, J Med Case Rep 2016 | 16 | Male | Características Cushing + weight gain + abdominal distension | Right and left trisegmentectomy | No | Living, 13 years |
| Khoshnam et al.2, Eur J Med Genet 2017 | 14 | Female | Características Cushing+ Hinchazón y dolor abdominal | Liver transplant (Lesión lóbulo hepático derecho) | Pre-op CTx | Living, |
| Tehseen et al.7, Pediatr Transplant 2017 | 13 | Female | Dolor abdominal + abdominal distension | Liver transplant (Lesión lóbulo hepático derecho) | Pre-op CTx | Living, 28 months |
| Cushing syndrome | ||||||
| Garg et al.8, Indian J Nucl Med 2017 | 8 | Female | Pancitopenia | Liver resection; liver transplant | Post-op CTx in recurrence | 2 months lymoh node and bone recurrence →CTx + Bone RT |
| Meletani et al.9, World J Gastroenterol 2017 | 31 | Male | Abdominal pain + weight loss | Right triseccionectomy + caudate resection + RF segment II | post-op CTx | Local recurrence after 6 months + p presacral nodule → chemoembolización + |
| CTx after (×4) | ||||||
| Éxitus: 15 months after surgery | ||||||
| Tsuruta et al.4, World J Surg Oncol 2018 | 20 | Male | Incidental | Right hepatectomy | post-op CTx | Éxitus: 2 months due to local recurrence and metástases in extrahepátic lymph nodes→ chemoembolización and RF |
| Olin et al.3, Int J Surg Case Rep 2020 | 15 | Female | Dolor abdominal | Right trisegmentectomy | No | Living, 12 months, sin Recurrence |
| Pérez, 2020 | 21 | Female | Asintomátic | Right triseccionectomy | No | Living, 6 months, sin Recurrence |
Post-op: postoperative; Pre-op: preoerative; CTx: chemotherapy; RF: radiofrecuency.
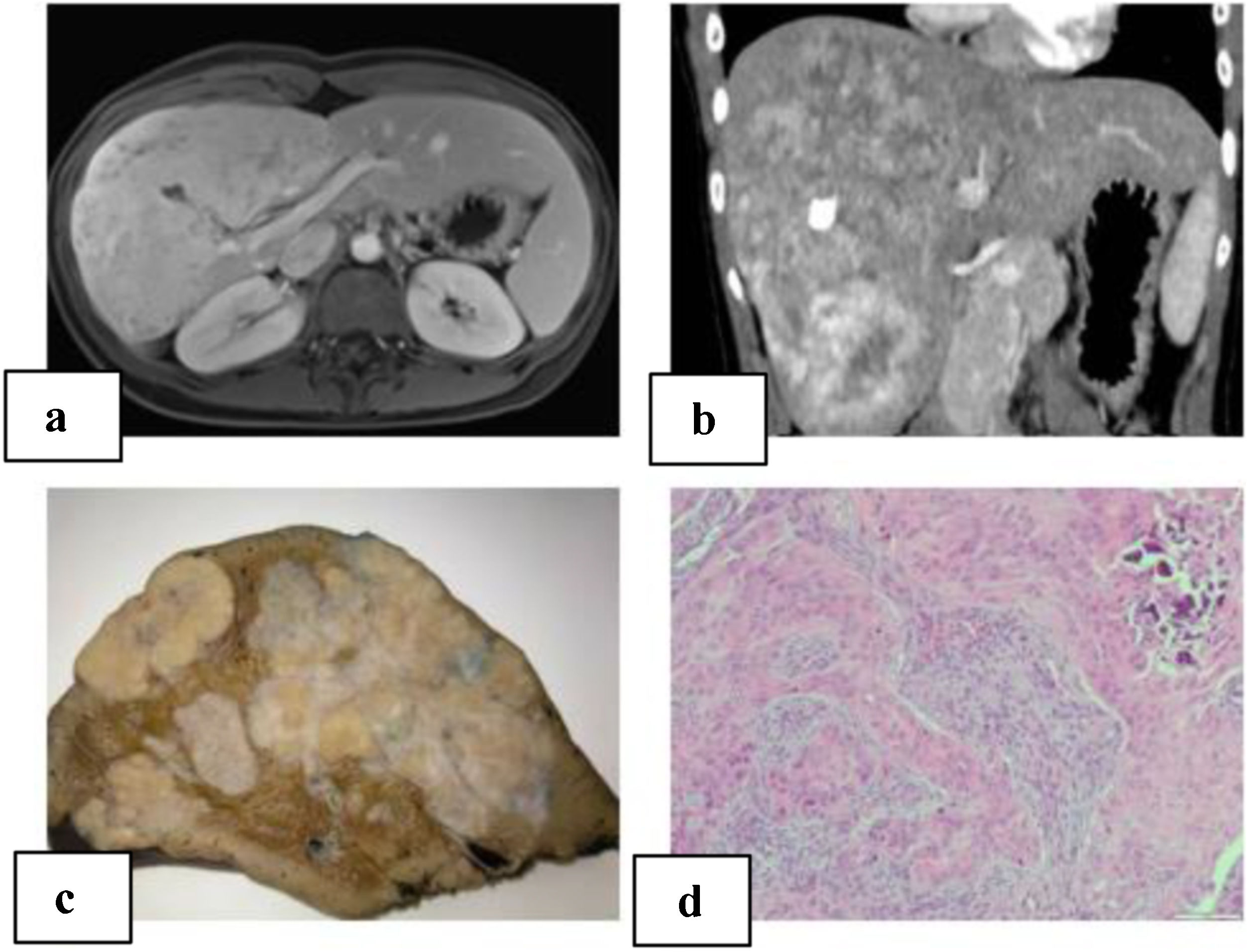
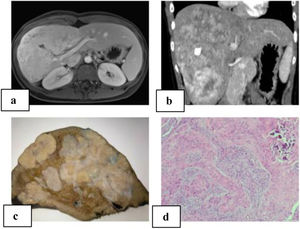
We present a 21-year-old patient with no relevant history. Follow-up lab work showed an altered liver profile (gamma glutamyl transpeptidase [GGT] 122 IU/L). Physical examination revealed hepatomegaly. Abdominal ultrasound identified a hyper-isoechoic focal lesion, apparently calcified, with a lobulated shape and limited vascularization. Magnetic resonance imaging (MRI) and computed tomography (CT) scan (Fig. 1A–B) showed a large, irregular, heterogeneous mass with multiple calcifications, with irregular enhancement in the arterial phase and washout in the portal phase. The initial diagnosis was fibrolamellar hepatocellular carcinoma (FHCC) in segments IV, V, VI, VII and VIII. Tumor marker levels (Alpha-fetoprotein, carcinoembryonic antigen and CA 19.9) were normal. Total liver volume was 1172.83 cc. The calculated residual volume percentages were: 42.2% for segments II-III (495.59 cc) and 48% for segments I-II-III (562 cc). Due to the suspicion of FHCC, surgery was indicated, and we performed right trisectionectomy by laparotomy as well as lymphadenectomy of the hepatic hilum and celiac trunk. The postoperative period transpired without incident, and the patient was discharged on the 7th postoperative day.
A) MRI of the liver: large, heterogeneous, polylobulated mass measuring 11.5 × 10 × 19 cm in segments IV, V, VI, VII and VIII with hypervascular uptake, with no clear washout in the portal and late phases, maintaining foci with no uptake at the central level, which coincided with the calcification on the CT scan; diffusion was slightly restricted; characteristics were compatible with fibrolamellar carcinoma; B) abdominal computed tomography: a large heterogeneous hepatic mass with irregular contour and heterogeneous density occupied segments IV, V, VI, VII and VIII, with multiple calcifications; irregular enhancement in arterial phase, and contrast washout in portal phase; C) macroscopic image: hepatectomy specimen measuring 24 × 17 × 19 cm and weighing 1500 g. It is almost entirely occupied by a 21 × 13 × 8 cm nodular whitish tumor that is in contact with the hepatic capsule and is away from the parenchymal resection margin; D) microscopic image with hematoxylin-eosin (10× magnification): proliferation of epithelioid and spindle-shaped cells with formation of osteoid; nests are observed with a central epithelial appearance, surrounded by spindle-shaped cells; calcified area.
The pathological study reported a whitish nodular tumor measuring 21 × 13 × 8 cm with multiple calcifications, areas of ossification and lymphadenopathies without neoplasm. Immunohistochemistry showed the cells were positive for Vimentin, Actin, WT-1, CD56, CD99, CD117 with Ki67:1% (Fig. 1C–D). These results were compatible with CNSET with free surgical margin, no perineural invasion or lymphovascular permeation.
The case was presented to the Oncology Committee, who decided to monitor the patient in an outpatient clinic without adjuvant chemotherapy because there is no clear benefit to justify adjuvant therapy. Six months after surgery, the patient remains asymptomatic and disease free. Follow-up studies included laboratory testing with liver panel and CT scan.
CNSET have been described in the literature with various terminologies: ossifying malignant mixed epithelial and stromal tumor, ossifying stromal epithelial tumor, and desmoplastic nested spindle-cell tumor of the liver1. It was Markhouf who proposed the term CNSET because it incorporates all the characteristics of the tumor.
Despite their exceptional nature, we know that they present more frequently in young (≤20 years) or pediatric patients (77%) and females (70%) (Table 1). They are usually located in the right hepatic lobe (64%), and in 40% of cases their diagnosis is incidental (Table 1). The association of CNSET with hormonal alterations is notable (36%): Cushing syndrome or cushingoid symptoms (25%)5,7, Klinefelter syndrome4 and oral contraceptives. Some authors have tried to link the development of these tumors with hormonal alterations, but this has not been proven. In four patients, it was associated with Beckwith-Wiedemann syndrome (overgrowth syndrome with increased risk of developing cancer)2,8. Our case did not present an association with these pathologies.
In terms of diagnosis, the lab analysis highlights normal liver function as well as the usual tumor markers1,3, although elevated GGT has been described in some cases5, including ours. On CT and MRI, it is characteristic to observe a large, well circumscribed, heterogeneous mass with dense calcification and macrolobulated margin1,3,4,6. The most common radiological differential diagnosis is hepatoblastoma, followed by FHCC. Confirmation of the diagnosis by preoperative biopsy has been described3. Geramizadeh published low profitability in a cohort of 12 patients with CNSET, where the diagnosis was reached in only three cases (25%). At our hospital, the use of liver biopsy is unusual and only reserved for uncertain diagnoses.
The standard treatment of CNSET is surgical resection, obtaining free margins1,3–5. The technique used has varied from partial resections to liver transplantation, which occurred in eight cases, depending on the extension and location of the tumor (Table 1).
Chemotherapy (CTx) was used in 38% of the cases of the series analyzed. Five cases received preoperative CTx, showing no effects of reduction or necrosis in the imaging tests or in the surgical specimens. Twelve patients received postoperative CTx, 50% as treatment for postoperative recurrence (Table 1). The regimens administered were similar to those for sarcoma or hepatoblastoma4–6. Today, its role in the prevention or treatment of recurrence is unclear1,4,5. Three were treated with radiofrequency (RF)4, and only Makhlouf describes a successful result with this therapy.
80% (35/44) of the patients did not experience disease recurrence. This occurred in nine patients with an interval from two months to 11 years4,8,9. Most recurrences were local, but they were found in several locations: lymph nodes, lungs or bone (Table 1). The follow-up described in the literature varies from two months to 22 years. In total, 7% of patients died from the disease, and 14% are living with recurrence (Table 1).
The prognosis is uncertain, but growth is usually slow, with low malignant potential1,4,5. Most cases have long-term survival after resection4 and often remain recurrence-free if the resection margins are negative3. A close and sustained follow-up is necessary, with lab work and imaging studies due to the risk of long-term recurrence2–5.
Please cite this article as: Pérez Reyes M, Sánchez Pérez B, León Díaz FJ, Santoyo Villalba J, Santoyo Santoyo J. Tumor calcificante en nidos epitelial-estromal: neoplasia hepática excepcional. Cir Esp. 2021;99:543–547.