Non-valvular atrial fibrillation (NVAF) is the most common arrhythmia in our setting. Its most serious complication is cardioembolic stroke due to the disability and mortality involved. The standard treatment for this arrhythmia is anticoagulation with vitamin K antagonists and oral anticoagulants (OAC), although the main disadvantage of these drugs is the increased risk of hemorrhage. More than 90% of thrombi of the left atrium are located inside the left atrial appendage (LAA), which has led to the creation of devices that close it to prevent cardioembolic risk. Percutaneous closure of the LAA is still a technique in its early stages, and its indication is currently reserved for patients with NVAF and contraindications to anticoagulant therapy.
Our patient is a 67-year-old male, hypertensive, and ex-smoker with NVAF but no structural heart disease and a high risk for embolism, treated with Edoxaban®. He presented an intraparenchymal hematoma in the left hemisphere as a complication, so a Watchman® left atrial appendage closure device was implanted. During the follow-up transesophageal echocardiogram performed one month later when the patient was completely asymptomatic, migration of the device was observed. Computed tomography was performed, and the device was found in the infrarenal abdominal aorta.
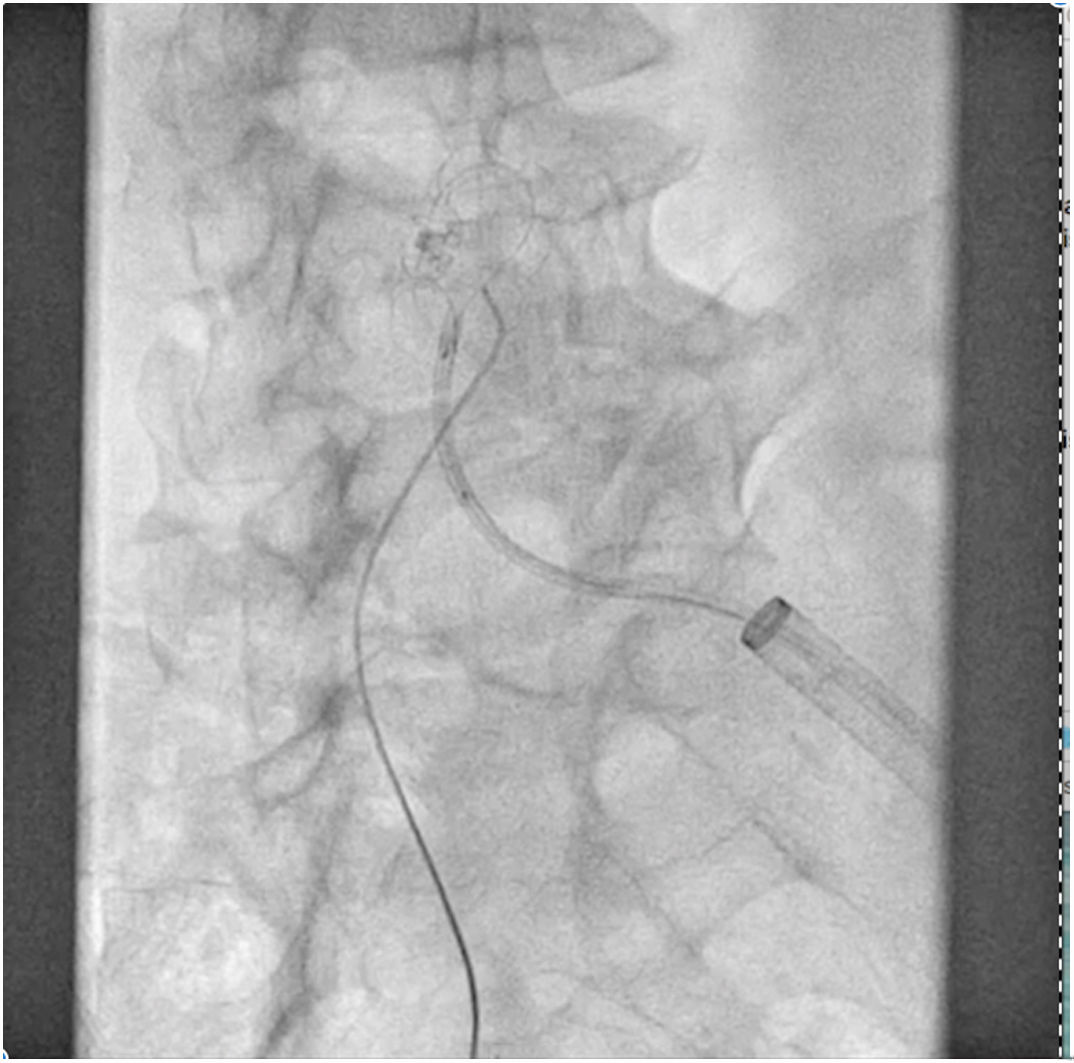
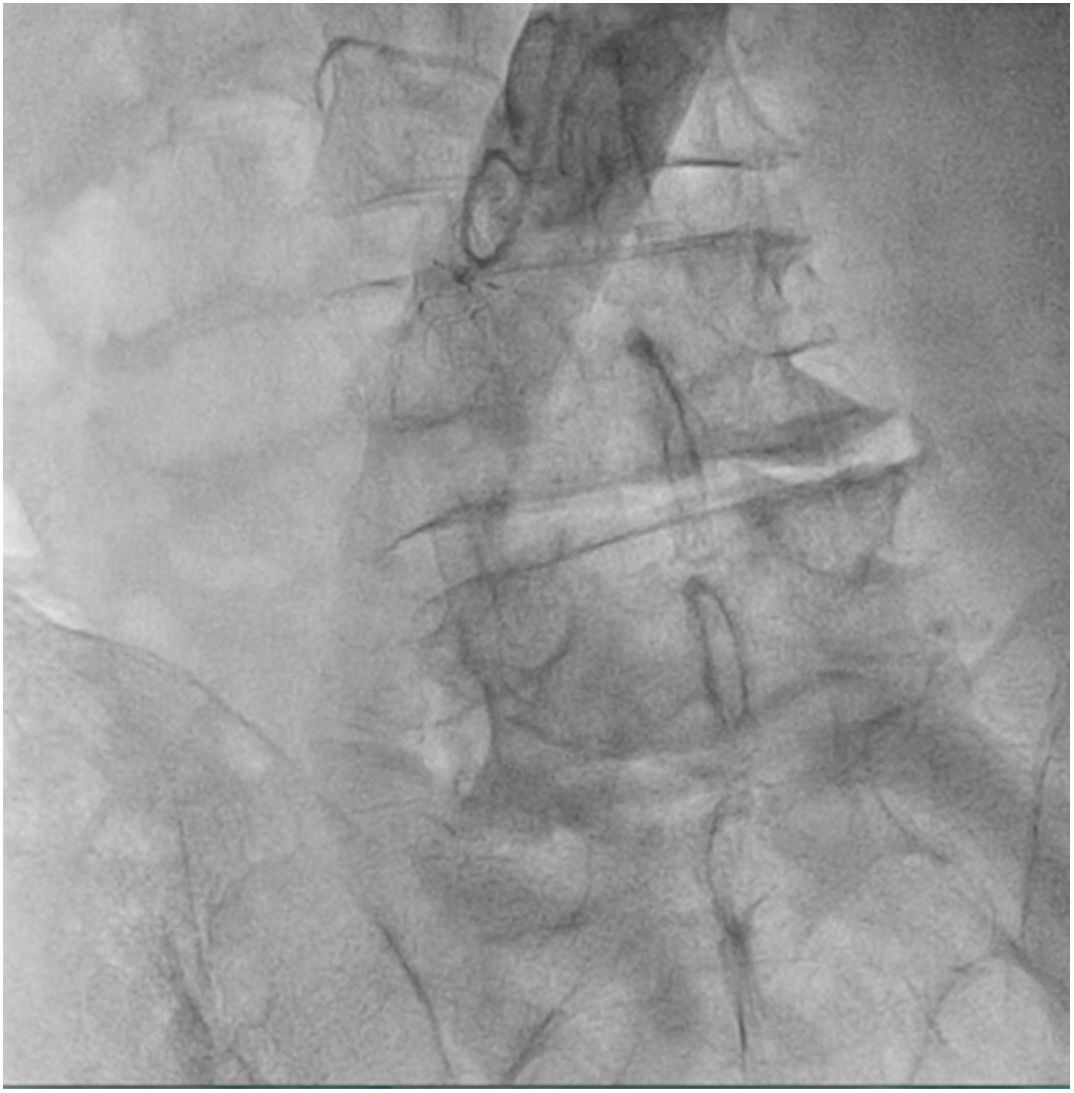
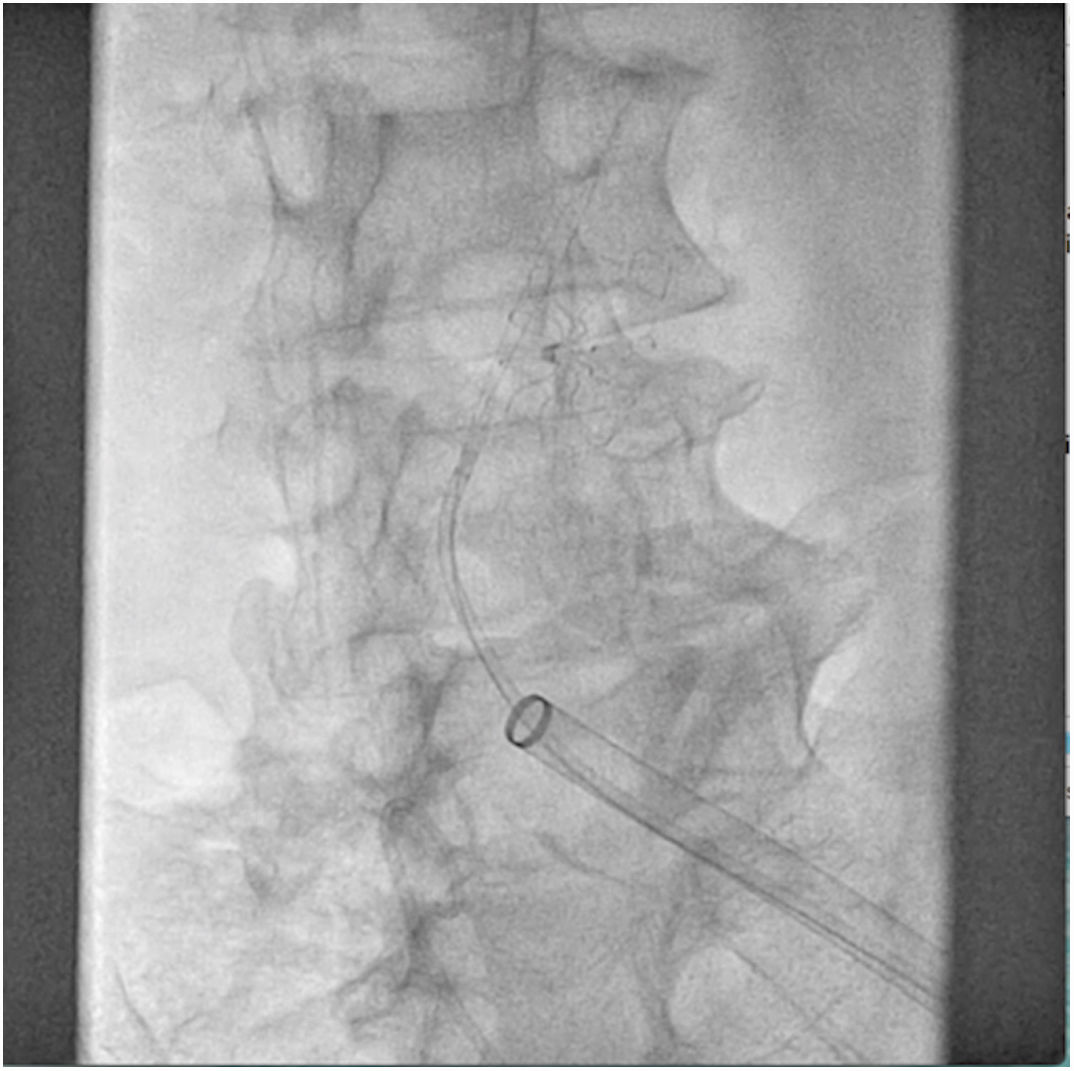
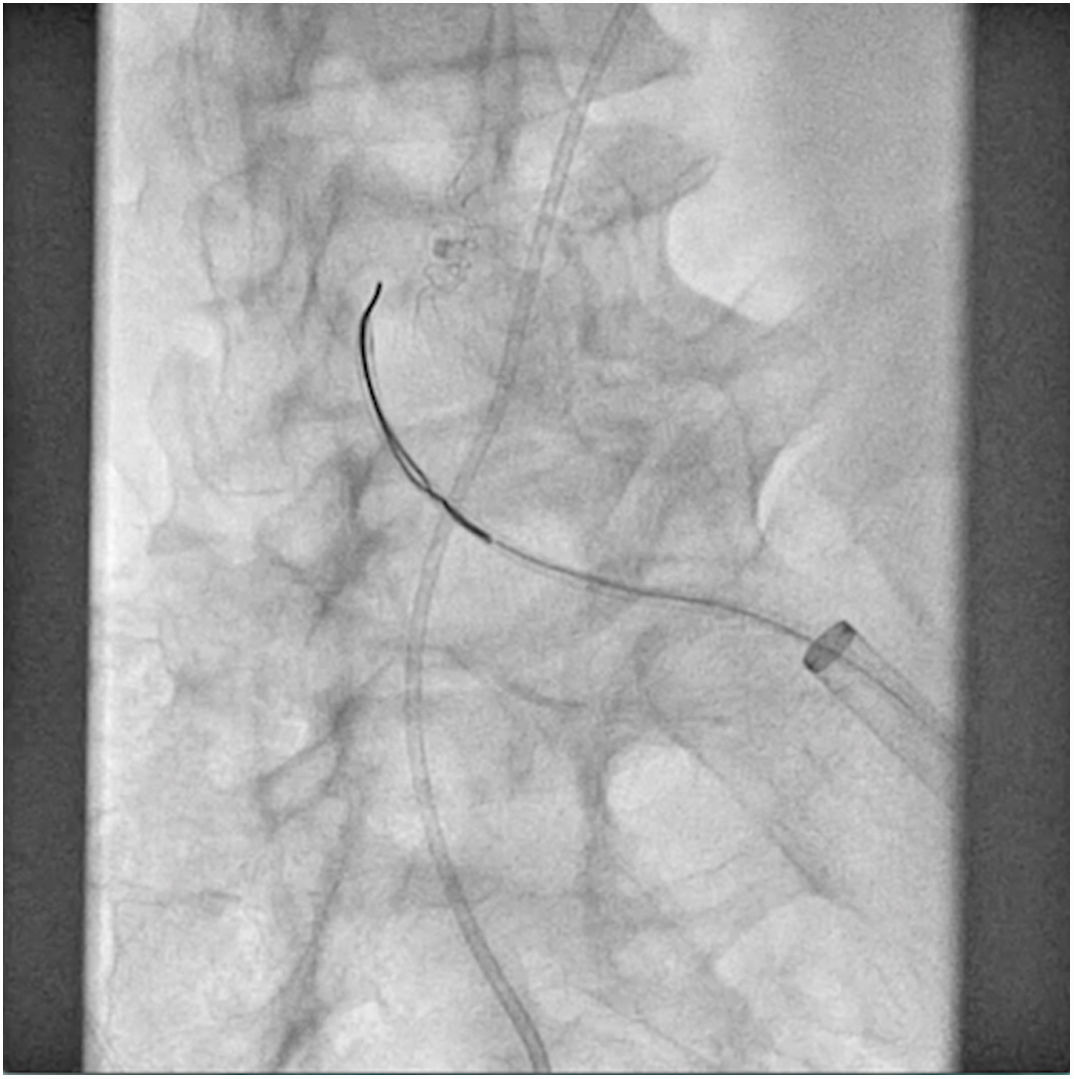
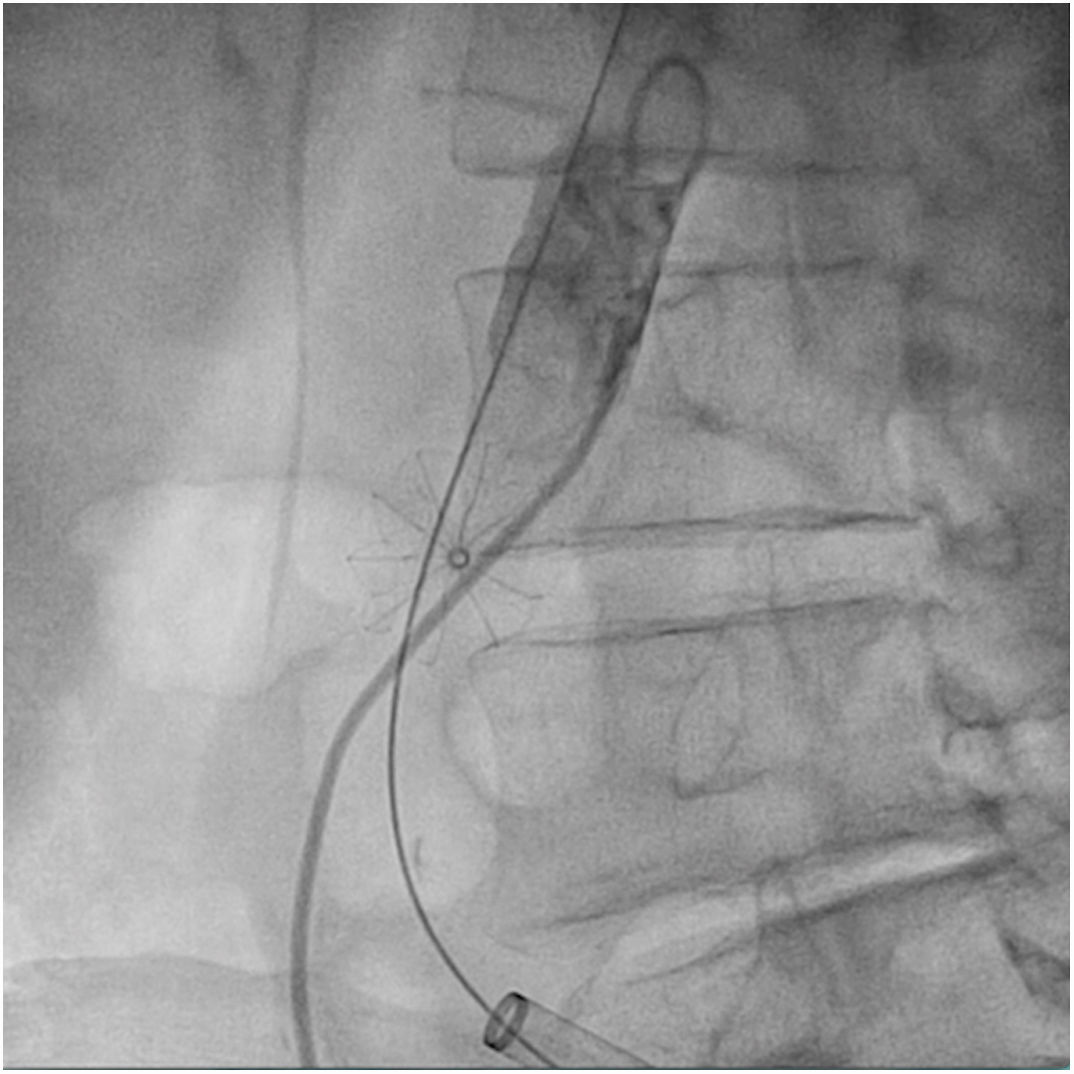
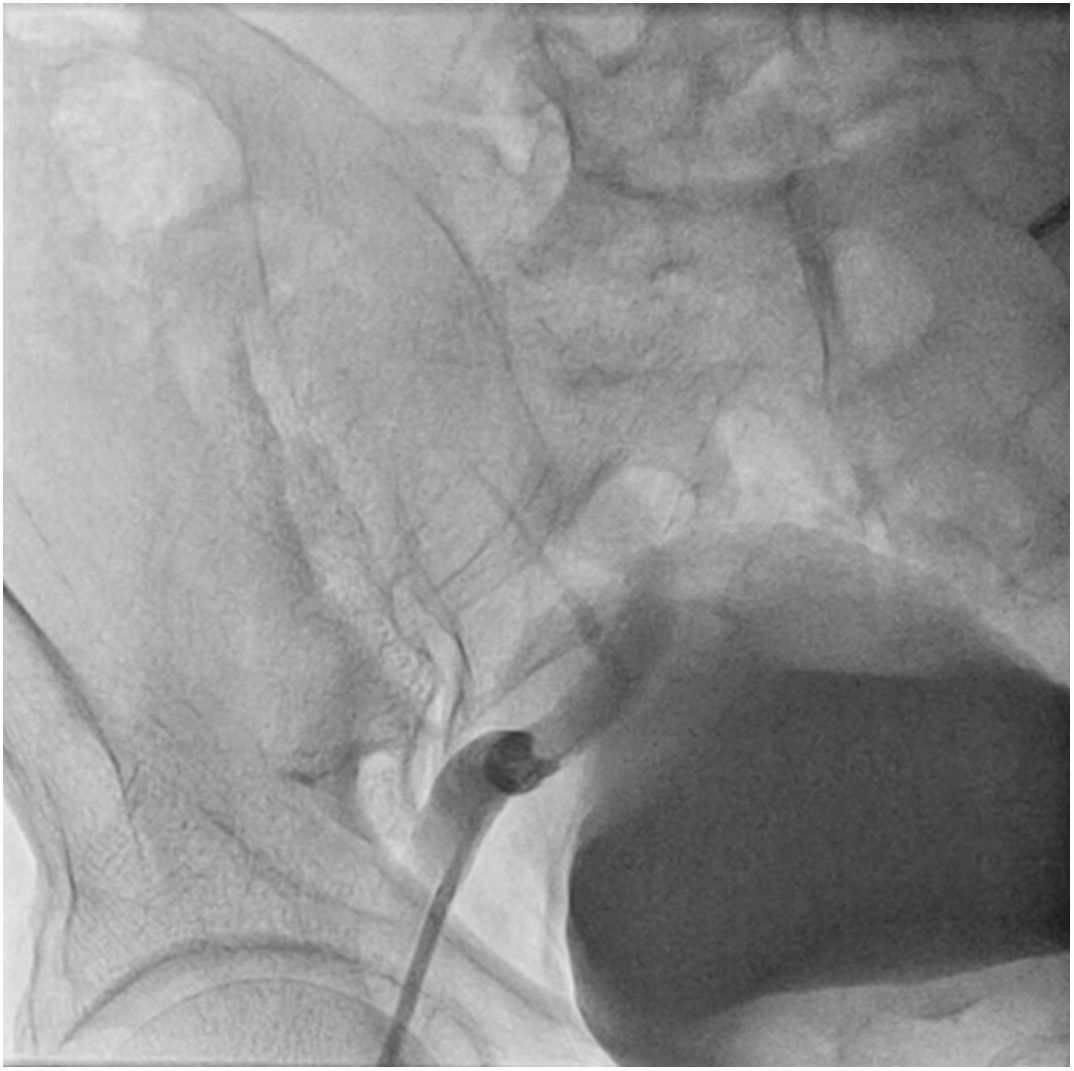
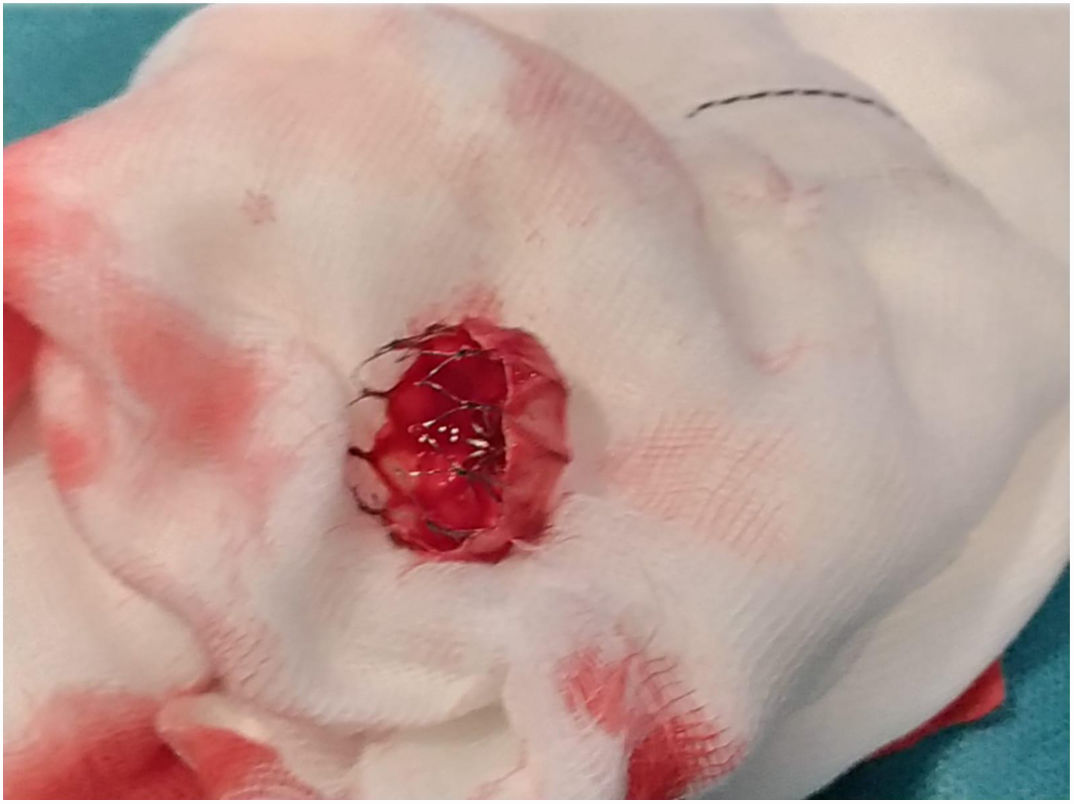
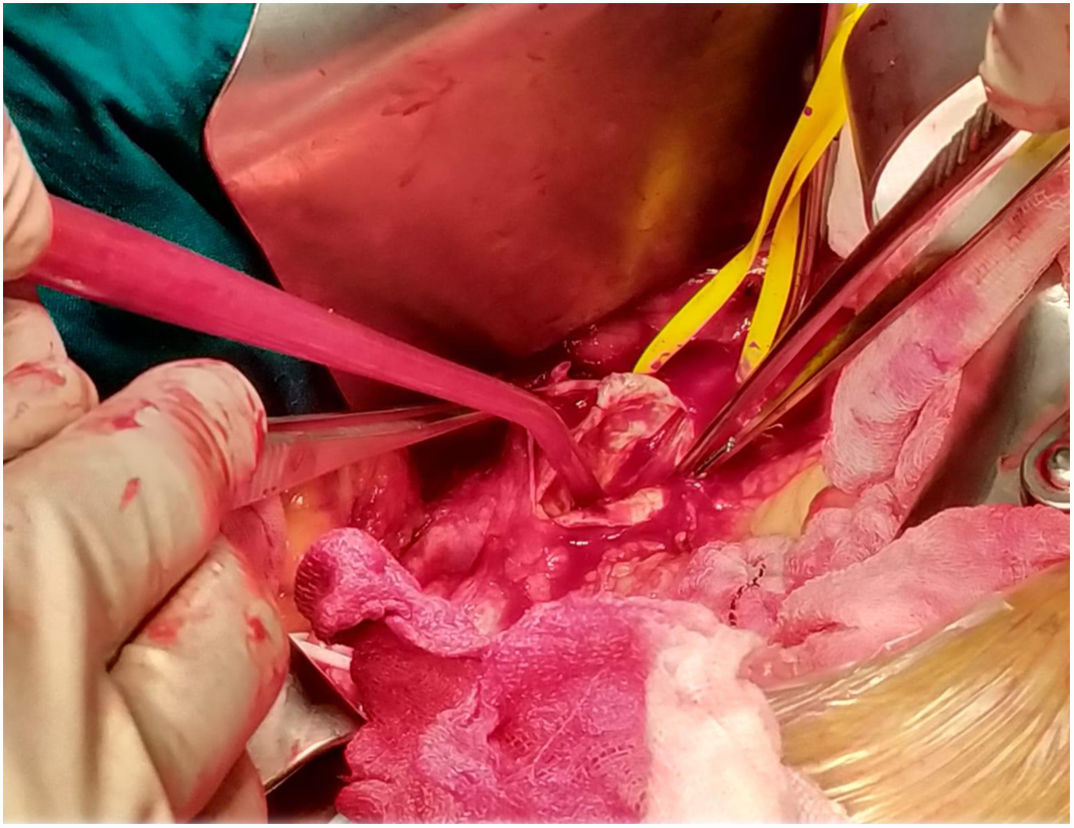
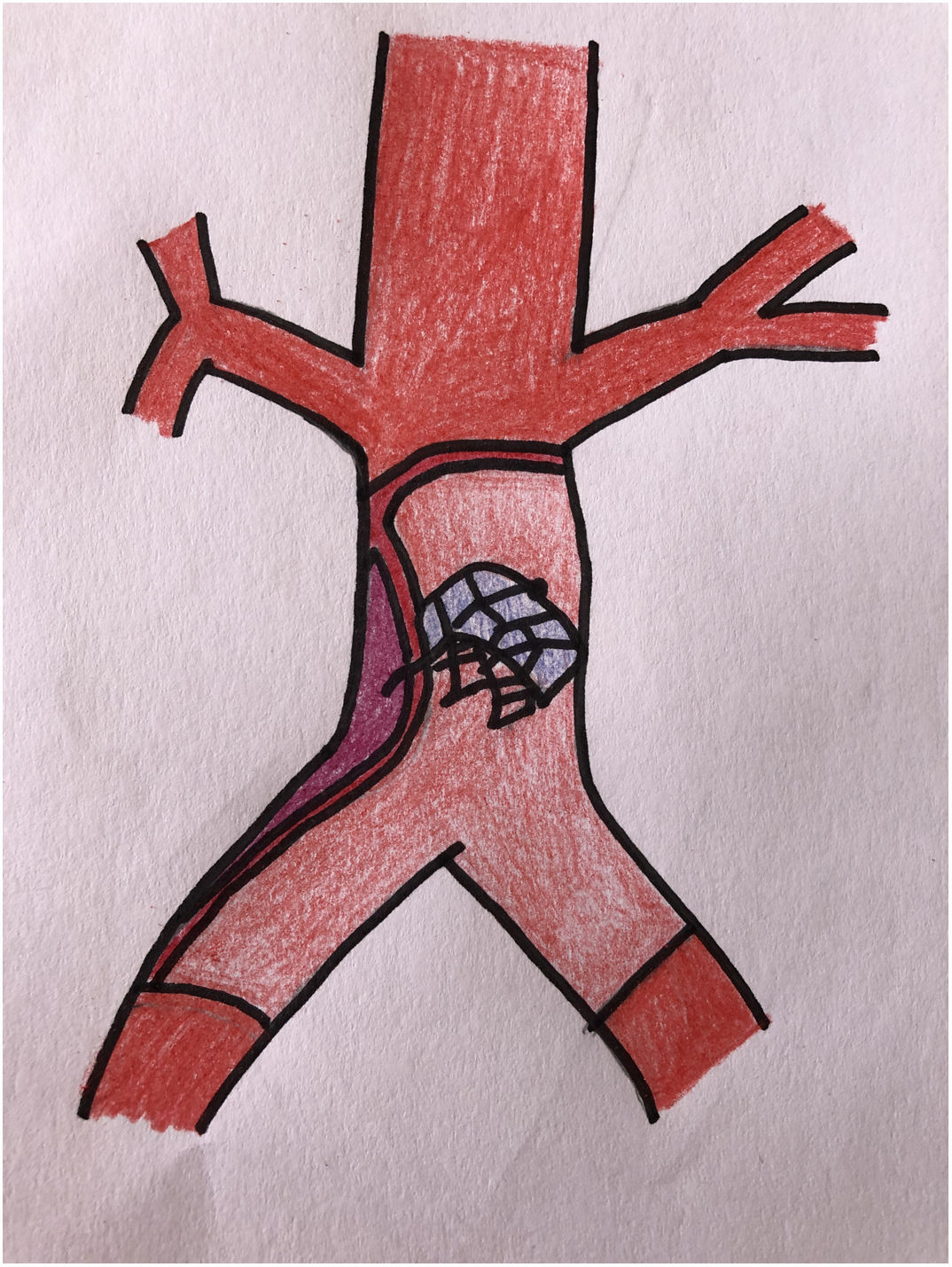
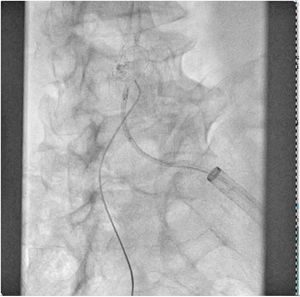
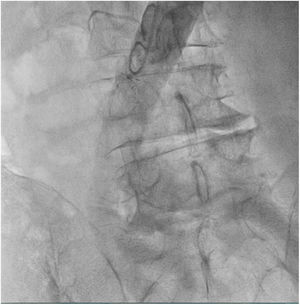
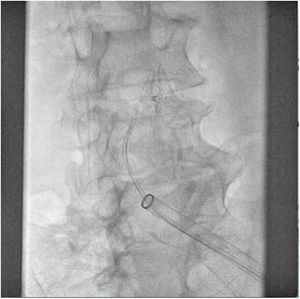
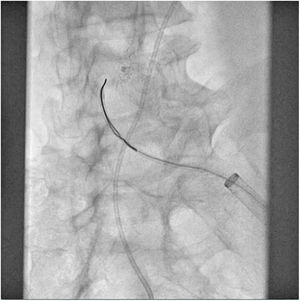
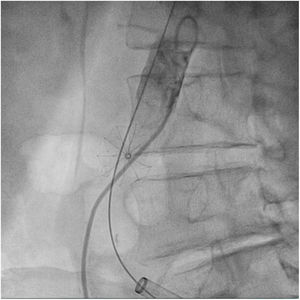
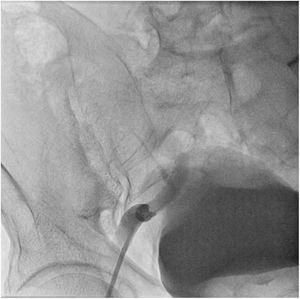
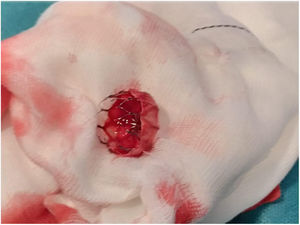
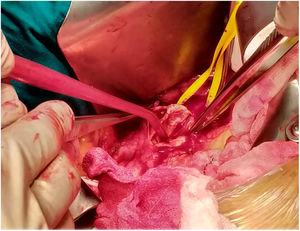
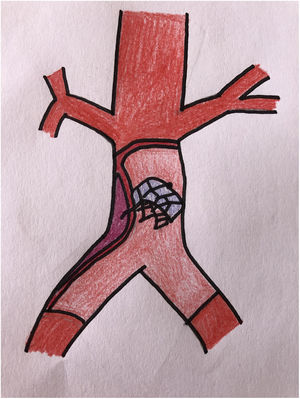
The patient was transferred to the hemodynamic unit, where we attempted to extract the device. Different 10-mm, 25-mm and 30-mm loop catheters were used, as well as manufactured loops with long and short guidewires, in an attempt to mobilize the device using different types of vascular access (bifemoral access 6 and 18 Fr, and radial 6 Fr) (Figs. 1–4). As a complication, abdominal aortic dissection occurred with extension to the right common iliac artery (Figs. 5 and 6) and total loss flow in the ipsilateral lower extremity with signs and symptoms of acute arterial ischemia. The patient was immediately transferred to the angiology and vascular surgery operating room. Infrarenal abdominal aorta dissection was performed via midline laparotomy with longitudinal aortotomy at the site of the foreign body, where the migrated device was observed to be anchored to the intima-media of the arterial wall. This was extracted, and the aortotomy was then closed with a bovine pericardial patch at the dissection to correct the defect (Figs. 7–9). The patient left the operating room with bilateral pedal pulse and excellent distal perfusion.
Given the difficulty to remove the device endovascularly, and due to the complication that occurred during the procedure, extraction by open surgery could be considered a first treatment option.
It is possible that the sinus rhythm ‘jumps’ that the patient experienced were the cause of the migration of the device, which should lead us to consider another possible therapeutic option, such as the implantation of a pacemaker.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Manosalbas Rubio IM, Martín Cañuelo JJ, Galera Martinez MC, García Turrillo E, Rodríguez Piñero M. Extracción aórtica de dispositivo Watchman® migrado. Cir Esp. 2021;99:243–245.