Enhanced recovery after surgery (ERAS) has demonstrated in colorectal surgery a reduction in morbidity and length of stay without compromising security. Experience with ERAS programmes in pancreatoduodenectomy (PD) is still limited. The aims of this study were first to evaluate the applicability of an ERAS programme for PD patients in our hospital, and second to analyse the postoperative results.
MethodsA retrospective study using a prospectively maintained database identified 41 consecutive PD included in an ERAS programme. Key elements studied were early removal of tubes and drainages, early oral feeding and early mobilisation. Variables studied were mortality, morbidity, perioperative data, length of stay, re-interventions and inpatient readmission. This group of patients was compared with an historic control group of 44 PD patients with a standard postoperative management.
ResultsA total of 85 pancreatoduodenectomies were analysed (41 patients in the ERAS group, and 44 patients in the control group. General mortality was 2.4% (2 patients) belonging to the control group. There were no statistical differences in mortality, length of stay in intensive care, reoperations, and readmissions. ERAS group had a lower morbidity rate than the control group (32% vs. 48%; P=.072), as well as a lower length of stay (14.2 vs. 18.7 days). All the key ERAS proposed elements were achieved.
ConclusionsERAS programmes may be implemented safely in pancreaticoduodenectomy. They may reduce the length of stay, unifying perioperative care and diminishing clinical variability and hospital costs.
La rehabilitación multimodal precoz (RMP) ha demostrado en la cirugía colorrectal una reducción de la morbilidad y de la hospitalización sin comprometer la seguridad de los pacientes. La experiencia de la RMP en la duodenopancreatectomía cefálica (DPC) es más limitada. Los objetivos de este estudio fueron analizar la aplicabilidad de un programa RMP en los pacientes intervenidos mediante una DPC en nuestro medio y evaluar los resultados postoperatorios.
MétodosEstudio retrospectivo utilizando una base de datos prospectiva de 41 pacientes a los que se realizó DPC y fueron incluidos en un programa de RMP. Se evaluaron 3 elementos clave: retirada precoz de sondas y drenajes, ingesta oral y movilización precoz. Las variables analizadas fueron la mortalidad, morbilidad, datos perioperatorios, estancia hospitalaria, reintervenciones y reingresos. Este grupo de pacientes fue comparado con un grupo control de 44 pacientes consecutivos, en los que se realizó una DPC con manejo postoperatorio estándar.
ResultadosSe estudió a 85 pacientes intervenidos con DPC (41 pacientes en el grupo RMP y 44 pacientes en el grupo control). La mortalidad global fue del 2,4%: 2 pacientes pertenecientes al grupo control. No encontramos diferencias significativas en la mortalidad, ingreso en Reanimación, reintervenciones ni reingresos. El grupo RMP presentó una morbilidad menor que el grupo control (32 vs. 48%; p=0,072), y una estancia hospitalaria menor (14,2 vs. 18,7 días; p=0,014). Todos los elementos clave propuestos fueron conseguidos.
ConclusionesLa RMP en la DPC puede implantarse con seguridad en nuestro medio. Permite unificar los cuidados perioperatorios, disminuir la variabilidad clínica y la estancia media y como consecuencia, el coste hospitalario.
As a result of the application of multimodal rehabilitation protocols in the area of colorectal surgery, morbidity has been reduced along with hospital stay duration and hospital expenses. Furthermore, patient satisfaction has improved.1–3 Implementation of these protocols in pancreaticoduodenectomy (PDT) is challenging due to its complexity and high morbidity rate.4 In recent years, mortality due to PDT has decreased to levels below 5% due to the evolution of surgical techniques, improvements in perioperative care and the treatment of patients in high volume centres.3,5,6 Because of the inflammatory and catabolic reaction produced following PDT, the application of a structured and multimodal protocol for the reduction of perioperative stress may be a useful tool to achieve objectives similar to those obtained in colorectal surgery. The objectives of this study are: 1) To determine if an early multimodal rehabilitation programme (EMR) for PDT is applicable in our setting. 2) To assess the possibility of improving results in terms of morbidity, mortality and length of hospital stay.
MethodsIn January 2011 we developed the EMR protocol for PDT (Table 1). Between January 2011 and January 2014, 41 consecutive PDTs were included in the EMR programme. The following key elements have been evaluated: (1) early removal of tubes and drains; (2) early oral intake and (3) early mobilisation. The results of this group of patients were compared with a historical control group consisting of 44 patients who had undergone surgery between January 2005 and December 2010. These were patients where a PDT was performed with standard postoperative management. All data were collected from a prospective database including pancreatic resections which have been performed consecutively in our centre.
Early Multimodal Rehabilitation Programme for PDT Protocol.
| Day 0 Surgery |
| • Admission on the same day as surgery |
| • Fasting condition: liquids 2h; solids 6h before surgery |
| • No preparation of the colon |
| • Antibiotic prophylaxis (amoxicillin clavulanate) |
| • Intermittent compression stockings |
| • Thoracic epidural c.: 0.25% levobupivacaine infusion intraoperative |
| • Normothemia with convection blankets |
| • Monitoring: CBP; invasive blood pressure; oesophageal temperature; muscle relaxation; anaesthetic depth; NG tube |
| • Standard IV fluid therapy according to CBP and diuresis |
| • Transfusion criteria: Hg 7.5g/dl, adjusting to patient morbidity |
| • Preventive analgesia: Paracetamol+dexketoprofen+tramadol |
| • Prophylaxis for nausea/vomiting: ondansetron+dexamethasone |
| • Drains (2) |
| • Extubation in the operating room |
| • Stay in postoperative resuscitation unit: 24h, except complications |
| Day 1 |
| • Respiratory therapy; compression socks |
| • Epidural PCA: levobupivacaine 0.125%+fentanyl (2μg/ml): 5ml/h |
| • NSAIDs+paracetamol+morphine rescue |
| • NG tube removal (if volume <400ml in 24h) |
| • Total parenteral nutrition (TPN) |
| • Start sips of cold water |
| Day 2 |
| • Liquid diet (water-infusions) |
| • Seated position for one hour in the morning and in the afternoon |
| • Personal hygiene in bed |
| Day 3 |
| • Liquid diet (stocks) |
| • Determination of amylase in drains |
| • Remove urinary catheter |
| • Epidural PCA: decrease to 3ml/hour |
| • Seated position for two hours in the morning and in the afternoon |
| • Personal hygiene in the toilet |
| Day 4 |
| • Crushed foods without fat (if tolerance) |
| • Removal of epidural catheter |
| • NSAIDs+paracetamol+morphine rescue |
| • Remove drains (if appropriate) |
| • Start ambulation |
| Days 5–6 |
| • Easy-to-chew fat-free diet |
| • Decrease parenteral nutrition (if oral tolerance is good) |
| • Remove drains (if appropriate) |
| • Ambulation |
| Day 7 |
| • Remove central line and parenteral nutrition (if oral tolerance is good) |
| • Soft fat-free diet |
| • Criteria for hospital discharge |
The variables analysed were the American Society of Anaesthesiology (ASA), probe and drain removal, intestinal transit, oral intake, seated position/ambulation, stay at the postoperative intensive care unit, length of hospital stay, percentage of surgical reoperations and percentage of readmissions. Mortality and complications were followed up until hospital discharge or death of the patient. Readmissions were registered up to 30 days after admission. Postoperative complications were recorded according to Clavien-Dindo classification.7
Surgical TechniqueAll the interventions were performed by the same two surgeons. Resection included an antrectomy, lymphadenectomy of the hepatoduodenal ligament, celiac artery and the right side face of the superior mesenteric artery. The reconstruction in both groups was a double loop Roux-en-Y and end to side pancreaticojejunal anastomosis in 2 layers with a silicone tutor and end-to-side hepaticojejunostomy. Latero-lateral gastrojejunal anastomosis was retrocolic in the control group and antecolic in the EMR group, as described by Hartel.8 All venous and vascular resections were performed on the mesenteric-portal vein axis (MSV/P). Three types of vascular reconstructions were made according to the degree of vascular infiltration: (1) lateral suture MSV/P in cases of infiltration equal to or less than 25% of the circumference of the vein; (2) segmental resection with end-to-end autologous anastomosis in cases where infiltration was greater than 50% of the circumference and (3) replacement with prosthetic polytetrafluoroethylene (PTFE) in a case where the penetration of the portal vein was 3cm in length.
All the patients remained in the Postoperative intensive care Unit for at least 24h. Octreotide was administered only to patients with a high risk of pancreatic fistula (pancreatic duct ≤1mm, or soft pancreas). Two drains were placed: a sub-hepatic drain and another drain next to the pancreatic anastomosis. A portal Doppler was performed at 24h in all patients who had had a vascular resection. Removal of the drains in the EMR group was determined by measuring amylase in the liquid drainage on the third day following surgery. Prokinetic drugs were administered only in cases of delayed gastric emptying (DGE). The values of amylase levels in the drains were determined from the third day following surgery.
DefinitionsPancreatic FistulaPersistence in draining liquid amylase is greater than 3 times its highest value in plasma after the third day following surgery. The type of pancreatic fistula was classified as grade A, B or C according to the criteria of the International Study Group on Pancreatic Surgery (ISGPS).9
Delayed Gastric Emptying (DGE)Need for nasogastric tube (NGT) for over three days, or placement after the third post-operative day, and the absence of oral tolerance after the first week following surgery.10
Biliary FistulaPersistence of liquid in the drain bilirubin is greater than 3 times the highest value in plasma after the fifth day following surgery.5
Statistical AnalysisData are presented as mean±SE (95%), or as a number (%). Comparisons between groups were analysed using independent samples, such as the t-test or Mann Whitney test for continuous variables and Chi square or Fisher's exact test for categorical variables. A P value <.05 was considered statistically significant. Statistical analyses were performed with the software package SPSS® 16.0 (SPSS® Inc., Chicago, IL, USA).
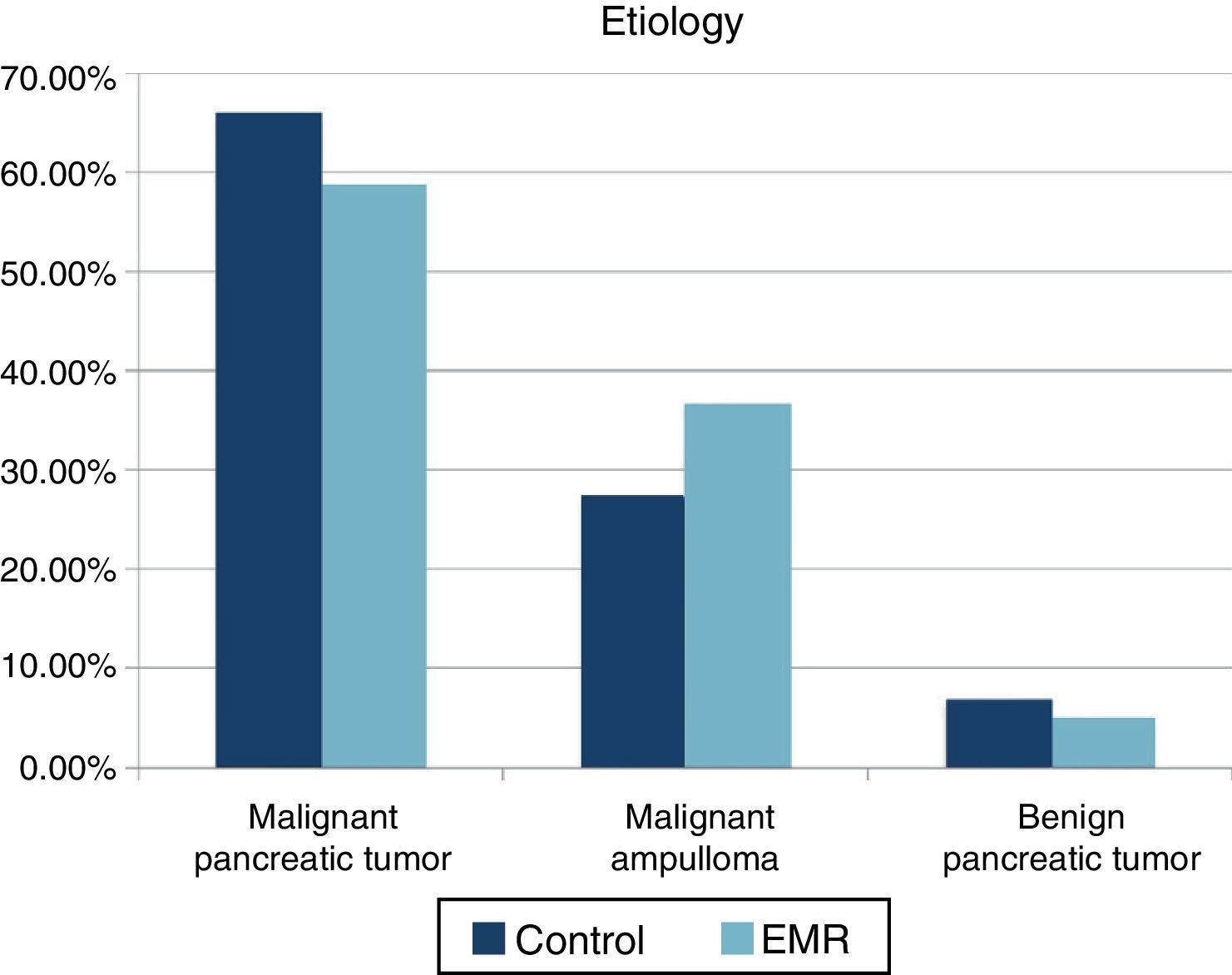
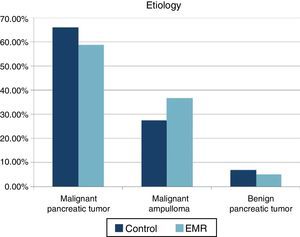
ResultsA total of 85 patients undergoing PDT (41 patients in the EMR group and 44 patients in the control group) were studied. The mean age was 66.7 (41–84) years in the control group and 61.3 years (44–80) in the EMR group. The ratio female/male was 39/61% in the control group and 41/59% in the EMR group. No statistically significant differences were recorded in the ASA between the two groups (ASA I: 52.9% in the control group and 47.1% in the EMR group). For ASA II- III the distribution was 51.4% and 48.6% respectively. Nor were there differences in classification by histology (Fig. 1). A lower incidence of preoperative biliary drainage was recorded in the control group (68.2% compared to 73.1%), although there were no significant differences.
Intraoperative and postoperative thoracic epidural analgesia was implemented in 98% of the patients. The intervention was of significantly shorter duration in the EMR group (5.9h) compared to the control group (6.4h) (P=.001). There were no significant differences in average intraoperative transfusion between both groups. In the postoperative period, however, the control group required a greater number of packed red blood cells than the EMR group (1.45 compared to 0.44; P=.001). No differences in the type of vascular resection were observed. Three side venous resections and one segmental resection with end to end anastomosis were performed in both groups. Venous reconstruction of the control group was made using PTFE prosthesis. The average stay in the intensive care unit was 2.8 days in the control group and 1.8 days in the EMR group (P=.95).
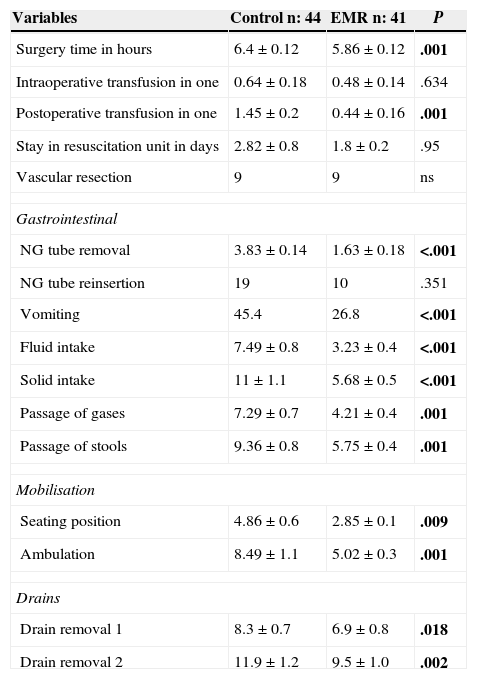
From the digestive point of view, what stands out is a shorter time of NG tube placement in the EMR group (1.6 compared to 3.8 days; P<.001), without a greater need for reinsertion and a lower vomiting rate after removal in the EMR group (26.8% compared to 45.4%; P=.029). Both intake of liquids and solids, and intestinal gas and stool transit occurred significantly earlier in the EMR group (P<.001) (Table 2).
Perioperative Variables.
| Variables | Control n: 44 | EMR n: 41 | P |
|---|---|---|---|
| Surgery time in hours | 6.4±0.12 | 5.86±0.12 | .001 |
| Intraoperative transfusion in one | 0.64±0.18 | 0.48±0.14 | .634 |
| Postoperative transfusion in one | 1.45±0.2 | 0.44±0.16 | .001 |
| Stay in resuscitation unit in days | 2.82±0.8 | 1.8±0.2 | .95 |
| Vascular resection | 9 | 9 | ns |
| Gastrointestinal | |||
| NG tube removal | 3.83±0.14 | 1.63±0.18 | <.001 |
| NG tube reinsertion | 19 | 10 | .351 |
| Vomiting | 45.4 | 26.8 | <.001 |
| Fluid intake | 7.49±0.8 | 3.23±0.4 | <.001 |
| Solid intake | 11±1.1 | 5.68±0.5 | <.001 |
| Passage of gases | 7.29±0.7 | 4.21±0.4 | .001 |
| Passage of stools | 9.36±0.8 | 5.75±0.4 | .001 |
| Mobilisation | |||
| Seating position | 4.86±0.6 | 2.85±0.1 | .009 |
| Ambulation | 8.49±1.1 | 5.02±0.3 | .001 |
| Drains | |||
| Drain removal 1 | 8.3±0.7 | 6.9±0.8 | .018 |
| Drain removal 2 | 11.9±1.2 | 9.5±1.0 | .002 |
In bold, intraoperative and postoperative variables.
After application of the EMR protocol, a faster postoperative mobilisation was achieved. The start of the seated posture changed from 4.8 days in the control group to 2.85 days in the EMR group (P=.018). Likewise, ambulation decreased from an average of 8.4 days in the control group to 5 days in the EMR group (P=.001). Regarding drain removal, differences were also significant. The two drains in the control group remained for an average of 8.3 and 11.9 days respectively, compared to 6.9 and 9.5 days in the EMR group (P=.018 and .002).
Overall mortality of the series was 2.4%, which corresponds to 2 patients in the control group. One case was of acute thrombosis of the expanded PTFE graft after resection of the portal vein, and the second patient died of nosocomial pneumonia. There were no deaths in the EMR group.
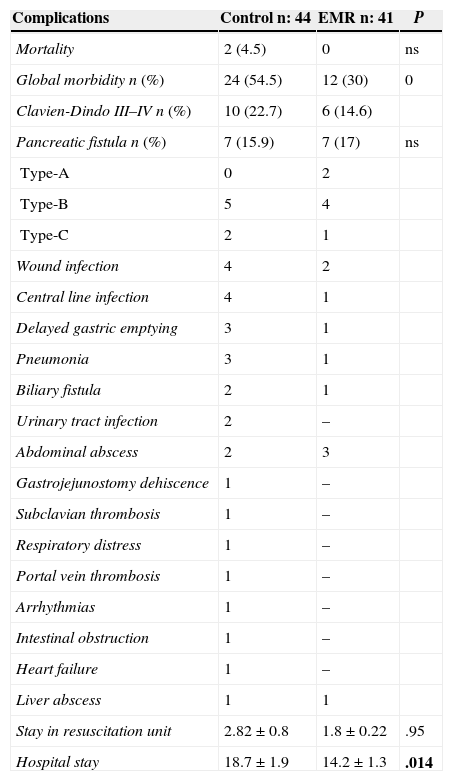
Overall mortality was 42.3% (54.5% in the control group and 30% in the EMR) (P=.029). The most frequent complications in the control group were pancreatic fistula (15.9%), DGE (6.6%) and wound infection (8.8%). The most frequent complications in the EMR group were pancreatic fistula (17%), intra-abdominal abscess (7.3%) and wound infection (4.9%). Complications grade III–IV of the Clavien-Dindo classification were 22.2% in the control group and 14.6% in the EMR group (Table 3). The reoperation rate was similar in both groups (11.3% compared to 12.1%), as was the readmission rate (9% compared to 9.7%). The hospital stay was significantly shorter in the EMR group (14.2 days), compared to 18.7 days in the control group (P=.014).
Morbidity and Mortality.
| Complications | Control n: 44 | EMR n: 41 | P |
|---|---|---|---|
| Mortality | 2 (4.5) | 0 | ns |
| Global morbidity n (%) | 24 (54.5) | 12 (30) | 0 |
| Clavien-Dindo III–IV n (%) | 10 (22.7) | 6 (14.6) | |
| Pancreatic fistula n (%) | 7 (15.9) | 7 (17) | ns |
| Type-A | 0 | 2 | |
| Type-B | 5 | 4 | |
| Type-C | 2 | 1 | |
| Wound infection | 4 | 2 | |
| Central line infection | 4 | 1 | |
| Delayed gastric emptying | 3 | 1 | |
| Pneumonia | 3 | 1 | |
| Biliary fistula | 2 | 1 | |
| Urinary tract infection | 2 | – | |
| Abdominal abscess | 2 | 3 | |
| Gastrojejunostomy dehiscence | 1 | – | |
| Subclavian thrombosis | 1 | – | |
| Respiratory distress | 1 | – | |
| Portal vein thrombosis | 1 | – | |
| Arrhythmias | 1 | – | |
| Intestinal obstruction | 1 | – | |
| Heart failure | 1 | – | |
| Liver abscess | 1 | 1 | |
| Stay in resuscitation unit | 2.82±0.8 | 1.8±0.22 | .95 |
| Hospital stay | 18.7±1.9 | 14.2±1.3 | .014 |
In bold, intraoperative mortality and postoperative complications.
In recent years, mortality has decreased for PDT, with rates below 5%, which is attributed to the evolution of surgical techniques to improve perioperative care and patient concentration in high volume centres.3,5,11–13 However, the morbidity rate of this procedure is still high, with rates of around 40%–50%, and a length of hospital stay of between 14 and 28 days.5,14,15 The implementation of the EMR programme in colorectal surgery has shown that it is safe and also that it improves the results, with a decreased morbidity and hospital stay duration, improvement in patient wellness and decreased clinical variability without increasing morbidity or mortality.1,2,16,17 While there are numerous publications of EMR in colorectal surgery, its application in pancreatic surgery has been more limited until recently, probably due to its greater complexity and morbidity. Given the inflammatory and catabolic reaction produced by the PDT, the implementation of a structured protocol designed to reduce perioperative stress is particularly interesting. Therefore, in recent years there have been several retrospective studies made with promising results in this area.3,14,15,18 In our country, Montiel et al.19 published a descriptive study of 82 patients, albeit without a control group.
In 2011, we decided to implement an EMR protocol for PDT in the Son Espases Hospital, to assess whether it may be implemented and the possibility of improving outcomes. All key elements proposed in the EMR programme were achieved. Significant differences in the removal of tubes and drains have been observed, along with the intake of liquids and solids and the mobilisation of patients. Premature withdrawal of the NG tube in the EMR group was followed by a significant decrease in postoperative vomiting (45.4% compared to 26.8%) and by a lower need for NG tube reinsertion (19% compared to 10%), although there were no significant differences. Early fluid intake did not increase the incidence of nausea or vomiting. Due to the early withdrawal of tubes and drains, and the epidural analgesia protocol, early mobilisation was achieved with the seated position on the second day and full ambulation after 5 days.
The multimodal approach to acute postoperative pain is one of the key factors in EMR programmes,20 which allows for lowering of the opioid dose and decreasing side effects. A recent meta-analysis21 showed that the use of epidural with general anaesthesia in major abdominal surgery reduces mortality, cardiovascular and respiratory complications. In addition, it accelerates intestinal transit, reduces postoperative ileus and the incidence of nausea/vomiting. PDT studies have shown better control of acute postoperative pain and fewer complications with the use of epidural analgesia.22 Moreover, the use of epidurals was associated with a lower average length of hospital stay.23
Drain use in pancreatic surgery is a routine practice. The incidence of fistulas is greater than in colon surgery, so its significance is different in this context. Although some authors suggest not using drains in low-risk fistula cases,24 most groups use them whilst recommending early withdrawal. This approach is useful for determinations of amylase in the fluid drainage.25–27
There has been a lower transfusion rate and shorter duration of the intervention in the EMR group, which could be attributed to greater experience of the surgical team. Most studies report a lower rate of complications in the EMR group.6,28 In our series, the overall morbidity was significantly lower in the EMR group, although these data must be analysed with caution, since it can be influenced by the sample size. The incidence of pancreatic fistula, as in other series,15 remained with similar figures in the EMR group, in spite of the early oral intake. DGE occurs after PDT in 15%–35% of the cases.10,29–31 The most common cause is the presence of a pancreatic fistula, but other causes have been suggested, such as decreased plasma motilin after duodenal resection and possible vagal denervation by dissection and lymphadenectomy in the hepatoduodenal ligament and the celiac trunk.5 The incidence of DGE in our study was lower in the EMR group (6.6% compared to 2.4%), in line with the studies where this protocol was applied.5,15,32 The only specific measures we associated to decrease the incidence of DGE were early oral intake and performing antecolic gastrojejunostomy, previously described by the Heidelberg group.5 The efficacy of prokinetic drugs in pancreatic surgery is subject to discussion, and at our centre we use them only in cases where DGE has been established.
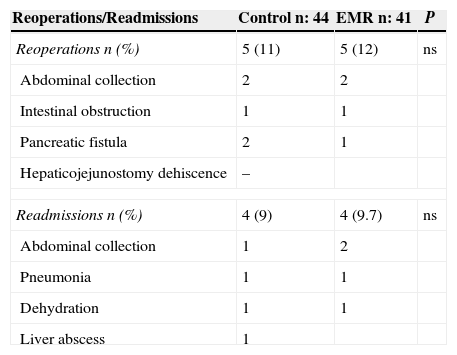
The reduction in hospital stay duration is one of the most important indicators of the EMR programmes.14,15,19,32 In the present study, the implementation of this programme helped to significantly reduce hospital stay (18.7 compared to 14 days). Reoperation rates and readmissions were similar in both groups (Table 4).
Reoperations and Readmissions.
| Reoperations/Readmissions | Control n: 44 | EMR n: 41 | P |
|---|---|---|---|
| Reoperations n (%) | 5 (11) | 5 (12) | ns |
| Abdominal collection | 2 | 2 | |
| Intestinal obstruction | 1 | 1 | |
| Pancreatic fistula | 2 | 1 | |
| Hepaticojejunostomy dehiscence | – | ||
| Readmissions n (%) | 4 (9) | 4 (9.7) | ns |
| Abdominal collection | 1 | 2 | |
| Pneumonia | 1 | 1 | |
| Dehydration | 1 | 1 | |
| Liver abscess | 1 | ||
Regarding limitations of this study, we suggest that its retrospective nature, lack of randomisation and comparison with a historical group could have resulted in a better experience and improvements in clinical practice in the second period. Another factor to consider is that the successful progressive implementation of EMR programmes for DGE will hinder the performance of randomised studies with a control group, for ethical reasons.
There has been an evolution of EMR programmes from the 1990s up to now. Of the 20 items initially proposed by Kehlet, current strategies are aimed at defining the key factors for achieving the objectives. A multivariate analysis in the Feroci study indicated five relevant factors for achieving good results: laparoscopy, the early removal of tubes and drains, early mobility and oral nutrition.33 One of the difficulties in interpreting the results of the EMR is the heterogeneity of the studies. A review of 5747 patients with colorectal cancer showed great variance in adherence to EMR programmes. Average length of stay and readmissions were two of the elements with the greatest variance.34
The future of the EMR programmes suggests several challenges35,36: the differentiation of clinical discharge from the actual discharge time is influenced by organisational and social health factors; the separation of surgical from medical complications; the standardisation of definitions to better define and compare results; and identification of the key factors that should be included in programmes to ensure their objectives. Finally, although the results of these programmes were more evident in patients without complications, some authors also suggest the application of EMR in patients who develop postoperative complications as they could also probably benefit from the principles of early rehabilitation.31,37
In conclusion, EMR programmes for DGE can be safely applied in our setting. They contribute to decreasing hospital stay duration and therefore hospital costs, and also help to unify perioperative care and reduce clinical variability. Despite the heterogeneity of the studies, the results are promising. Progressive implementation of these programmes will hinder the performance of randomised studies with a control group, for ethical reasons.
Conflict of InterestAll authors declare having read the article, agree with its conclusions and have no conflict of interest.
Our thanks to Dr. Jose Manuel Ramirez for his tireless work in the dissemination and implementation of the concepts of early multimodal rehabilitation in our country.
Please cite this article as: Morales Soriano R, Esteve Pérez N, Tejada Gavela S, Cuadrado García Á, Rodríguez Pino JC, Morón Canis JM, et al. Rehabilitación multimodal precoz: ¿podemos mejorar los resultados en la duodenopancreatectomía cefálica? Cir Esp. 2015;93:509–515.