El beneficio de las estatinas en la reducción de eventos coronarios se ha asociado con la repoblación por células musculares lisas (CML) de centros acelulares de placas de ateroma y su estabilización. Las LDL de la intima modulan el fenotipo de las CML e inducen desestabilización de las placas. Nosotros hemos demostrado que las LDL inhiben el remodelado vascular debido a un efecto sobre proteínas citoesqueléticas. Aquí, mediante una aproximación proteómica hemos estudiado si la rosuvastatina revierte los efectos de concentraciones aterógenas de LDL en las CML humanas.
Métodos y ResultadosCML tratadas con/sin 10μM rosuvastatina se incubaron en presencia/ausencia de 100μg/mL LDL (24h). Mediante 2DE y MALDI-ToF MS identificamos 39 proteínas no redundantes (52 spots) con expresión diferencial en CML tratadas con LDL. La rosuvastatina revirtió el efecto de las LDL en 13 de estas proteínas. Así, 4 proteínas disminuyeron su intensidad, 6 aumentaron y 3 proteínas multispot cambiaron su patrón proteómico. Estas proteínas se localizaron en el retículo endoplasmático, mitocondria, núcleo, membrana celular, citoesqueleto y citoplasma. La rosuvastatina afectó a proteínas involucradas en diversas funciones como regulación del citoesqueleto, actividad chaperona, metabolismo de carbohidratos, síntesis y plegamiento de proteínas, actividad cinasa, procesos redox, citoprotección y proliferación celular.
ConclusionesLos resultados demuestran que la rosuvastatina revierte el efecto de las LDL en CML a través de su efecto sobre proteínas funcionales, relevantes para el metabolismo y dinámica celular, lo que podría contribuir al efecto beneficioso de las estatinas en la estabilización de placas ateroscleróticas ricas en lípidos en la aterosclerosis humana.
The clinical benefits of statins in reducing coronary events have been related to repopulation of the acellular core of atheromatous plaques with vascular smooth muscle cells (VSMC) and plaque stabilization. Intimal low-density lipoproteins (LDL) have been associated with VSMC phenotypic modulation and plaque destabilization. We have recently reported that LDL impair vascular remodelling because of changes affecting cytoskeleton proteins. Here, we used a proteomic approach to study whether rosuvastatin reverses the effects induced by atherogenic concentrations of LDL in human VSMC.
Methods and ResultsVSMC treated with/without 10μM rosuvastatin were incubated in the presence/absence of 100μg/mL LDL for 24h. Using 2DE and MALDI-ToF MS, we identified 39 non-redundant proteins (52 spots) with differential expression in LDL-treated cells. Thirteen of these proteins were reverted to normal levels by rosuvastatin. Thus, four proteins showed decreased intensity, six showed increased intensity, and three multispot proteins changed their distribution pattern in the rosuvastatin group. These proteins were identified as components of endoplasmic reticulum, mitochondrion, nucleus, cell membrane, cytoskeleton, and cytoplasm. In LDL-VSMC, rosuvastatin affected proteins involved in a variety of functions including cytoskeleton dynamics, chaperone activity, carbohydrate metabolism, protein biosynthesis and folding, kinase activity, redox processes, cytoprotective effects, and cell proliferation.
ConclusionsOur results demonstrate that the reversing effects of rosuvastatin in LDL-enriched VSMC affect functional proteins with a role in cellular metabolism and dynamics, which might contribute to the beneficial effects of statins by stabilizing lipid-rich atherosclerotic plaques in human atherosclerosis.
Unstable human plaques often have high amount of infiltrated lipids and a relative paucity of smooth muscle cells (VSMC), which have been suggested to contribute to plaque rupture and consequently to acute coronary syndromes1
The phenotype of VSMC profoundly governs the properties and functions of the vessel wall. VSMC exhibit a high degree of plasticity which enable them to exhibit a wide range of different phenotypes in response to environmental and pathologic stimuli2. Current evidence suggests that intimal VSMC differ significantly from medial VSMC and as such, may have unique atherogenic properties that make them the ground for the initiation, progression, and complication of the plaques3,4.
LDL are the most atherogenic type of lipoproteins both in plasma and in vessel wall and retention of LDL in the arterial intima is a key event in atherosclerotic plaque formation5,6. Previous studies from our group demonstrated that LDL trigger changes on VSMC gene expression and phenotype leading to alterations in vascular function7–9. To this respect, we have recently demonstrated that LDL impair VSMC migration and wound repair after injury10 because of changes affecting cytoskeleton proteins involved in migration-kinetics10,11.
The inhibitors of 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase, statins, are cholesterol-lowering drugs that significantly reduce the presentation of cardiovascular events in patients either with or without previous coronary heart disease12–14. Clinical studies also suggest that statins may exert vasculoprotective effects that are independent of their cholesterol-lowering properties15. Among other, beneficial effects of statins include stabilization of atherosclerotic plaques16. Thus, analysis by magnetic resonance imaging (MRI) of aortic and carotid artery plaques of patients treated with simvastatin has shown that statins reduced the size of the lesions and the thickness of the arterial wall without changes in the lumen size17. Recent clinical studies report changes in carotid artery morphology in terms of increasing echogenicity and fibrous tissue content as an effect of statins18,19
To date, however, the molecular processes that directly relate LDL and statins with changes in VSMC phenotype are still poorly understood. Herein, we used two-dimensional gel electrophoresis, image analysis and mass spectrometry to identify effects of rosuvastatin on the proteome of LDL-loaded human coronary VSMC.
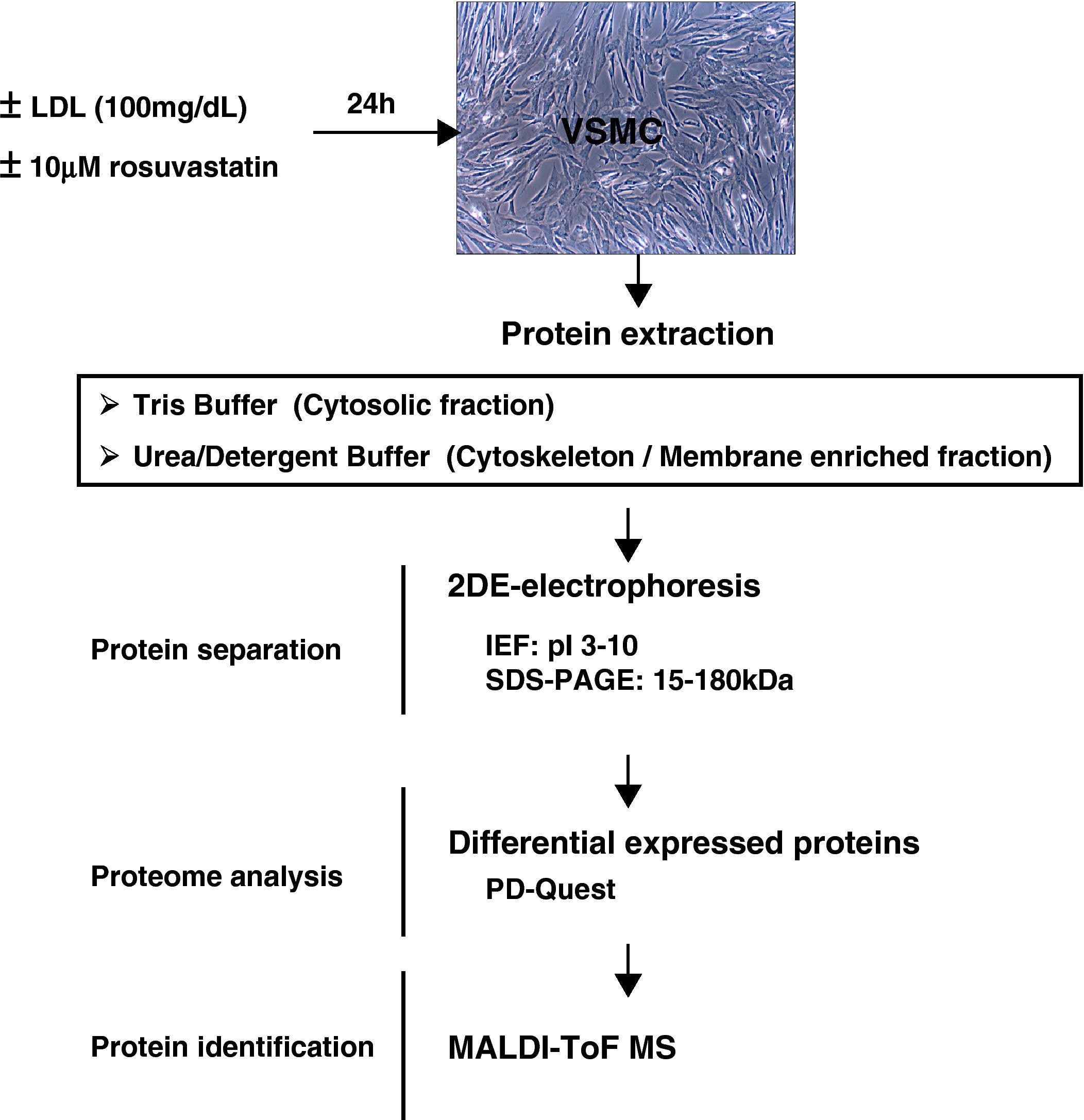
2Methods2.1Human coronary VSMC culture:Primary human VSMC were obtained by a modification of the explant technique from human non-atherosclerotic coronary arteries from hearts removed during transplantation surgery at the Hospital de la Santa Creu i Sant Pau, as previously described20. All procedures were approved by the Institutional Ethics Committee of the Hospital. VSMC were cultured in M199 supplemented with 20% (v/v) fetal calf serum (FCS), 2% human serum, 2mM L-glutamine, and antibiotics (100 IU/ml penicillin, 100μg/ml streptomycin). Cell culture media and reagents were from GIBCO/BRL (Invitrogen). Cells (from 3rd to 6th passages) were grown in 175cm2 plates and arrested at subconfluency with medium containing 0.4% FCS for 24hours. Thereafter, VSMC were incubated for 24hours in serumfree M199 media with/without 10 mM rosuvastatin in the presence or absence of 100μg/ml native LDL. Then, cells were washed with phosphate-buffered saline (PBS) and harvested in PBS containing 5mM EDTA, centrifuged and stored at –80°C until used.
2.2LDL preparationLDL (density 1.019 to 1.063g/mL) were obtained from pooled sera of normocholesterolemic volunteers after approval of the Institutional Ethics Committee. LDL were isolated and prepared by sequential ultracentrifugation, as described21. The content of protein (BCA protein assay; Pierce) and cholesterol (Cholesterol assay kit; Roche) in the LDL preparations were determined by colorimetric assays. The absence of contamination by other lipoproteins was determined by electrophoresis on agarose gels (Paragon Electrophoresis kit, Beckman). LDL used in the experiments were less than 48hours old. LDL had no detectable levels of endotoxin (Limulus Amebocyte Lysate test, BioWhittaker). LDL-oxidation was excluded by measuring thiobarbituric acid-reactive substances (TBARS). Human LDL, obtained as described above, did not contain detectable levels of thiobarbituric acid-reactive substances. Furthermore, human VMSC incubated with 10% FCS- M199 containing 100μg/mL LDL showed values under detection limit in the TBARS test through all the 24hours incubation-period.
2.3Protein ExtractionFrozen cell pellets were sequentially extracted based on differential protein solubility. Thus, a tris-soluble fraction and a urea-detergent soluble fraction were obtained. Briefly, samples were homogenized in 40 mmol/L tris-base buffer, incubated for 15minutes at room temperature (RT) and centrifuged at 16000 x g (20minutes). Protein pellets were washed once with tris-buffer and further extracted with a urea/detergent buffer (7mol/L urea; 2mol/L thiourea; 4% CHAPS; 40 mmol/L tris-base) for 15minutes at RT, as described above. Protein concentration was measured with 2D-Quant Kit (Amersham), as indicated by the manufacturer. Samples were divided into aliquots and stored at -80°C.
2.4Two-Dimensional Gel Electrophoresis (2-DE)Sample contaminants (salts, nucleic acids, lipids) were removed (2D-CleanUp Kit, GE HealthCare) and proteins separated by 2-dimensional electrophoresis (2-DE) as previously described10.
Isoelectric focusing (IEF) was performed using the Protean-IEF cell (BioRad). Extracts (120μg to 300μg) were loaded on 17-cm dry strips (pH 3-10 linear range, Bio-Rad) by active rehydration at 50V during 16hours. Proteins were then focused by progressive voltage increase up to 10000V within 5hours, limited by a maximum current of 50μA per g el, with a final step at 10000V until 70000 Volts Hour. After IEF, the strips were equilibrated with a reducing solution (50mM Tris-HCl buffer, pH 8.8, containing 6M urea, 2% SDS, 30% glycerol, and 2% DTT) and an alkylating solution (50mM Tris-HCl buffer pH 8.8, 6M urea, 2% SDS, 30% glycerol, and 2.5% iodoacetamide), for 15minutes. For the second dimension, strips were placed on top of 12% SDS-polyacrilamide gels. Electrophoresis was performed using an Ettan Daltssix System (GE HealthCare) at 40mA/gel. Protein spots in the gels were labelled by fluorescence with Flamingo (BioRad, fluorescence labelling), as described by the provider.
2.5Differential Image AnalysisGels were digitalized using a Typhoon Scanner (GE-HealthCare) and the resulting images were processed with the PD-Quest 8.0 2-DE software (Bio-Rad) for spot detection, quantification, and comparative analysis. Landmarks, which aid the gel-to-gel spot matching process, were also defined in the gels to increase the accuracy of the matching algorithm. Each spot was assigned a relative value (ppm) that corresponds to the single spot volume relative to the total volume of all spots present in the gel, following background extraction and removal of other artifacts. To accurately compare spot quantities between gels, normalization between gels was based on the total spot volume of all valid protein spots in each gel. The differentially expressed spots were identified based in a volume ratio fold-change of at least 1.5 (>1.5, <0.67).
2.6Protein Identification by Maldi-ToF-MS AnalysisProtein spots of interest were excised from 2-DE gels, washed (25mM Ambic), dehydrated (25mM Ambic/50% ACN followed by 100% ACN), dried, and enzymatic digested with one gel volume of sequence-grade modified porcine trypsin (Promega). Peptides from in-gel-trypsin digestion were desalted and concentrated by ZipTipU-C18 (Millipore) and mixed 1:1 with 5mg/mL α-cyano-4-hydroxy-cinnamic and spotted on a stainless steel mass spectrometry slide.
Protein identification was performed by peptide-mass fingerprinting using an Ettan MALDI-TOF Pro (matrix-assisted laser desorption/ionisation time-of-flight mass spectrometer, GE-Healthcare) operated in delayed extraction/reflecton mode, following the manufacturer's recommended procedure. MALDI-generated mass spectra were internally calibrated using Angiotensin-III (Ang-III) and Adrenocorticotropic Hormone (ACTH) peaks. Peak detection was performed with a centroid algorithm and using a mass range between 800 and 3500 m/z. A minimum quality value of 0.60 was considered. The monoisotopic cut off was set in 3000 m/z. Mass tolerance was 0.2 m/z and 1 m/z for monoisotpic and average peaks respectively.
Proteins were identified by peptide mass finger printing (PMF). To this aim, the peptide masses were searched against the National Center for Biotechnology Information (NCBI) non-redundant mammalian database using ProFound™ and Mascot search engines from MatrixScience selecting the SwissProt database. One missed cleavage per peptide was allowed and an initial mass tolerance of 1.2Da was used in all searches. Partial oxidation for methionine was assumed. For the present study, protein identification was based on measurements with a minimum coverage of 20%. Minimal expectation for valid identification was P<0.05.
2.7Statistical AnalysisResults are presented as mean±SEM or otherwise it is stated. Statistical significance for differences in spot intensity differences between groups was analyzed using the Student t-test and One-way ANOVA (R software environment; http://www.r-project.org).
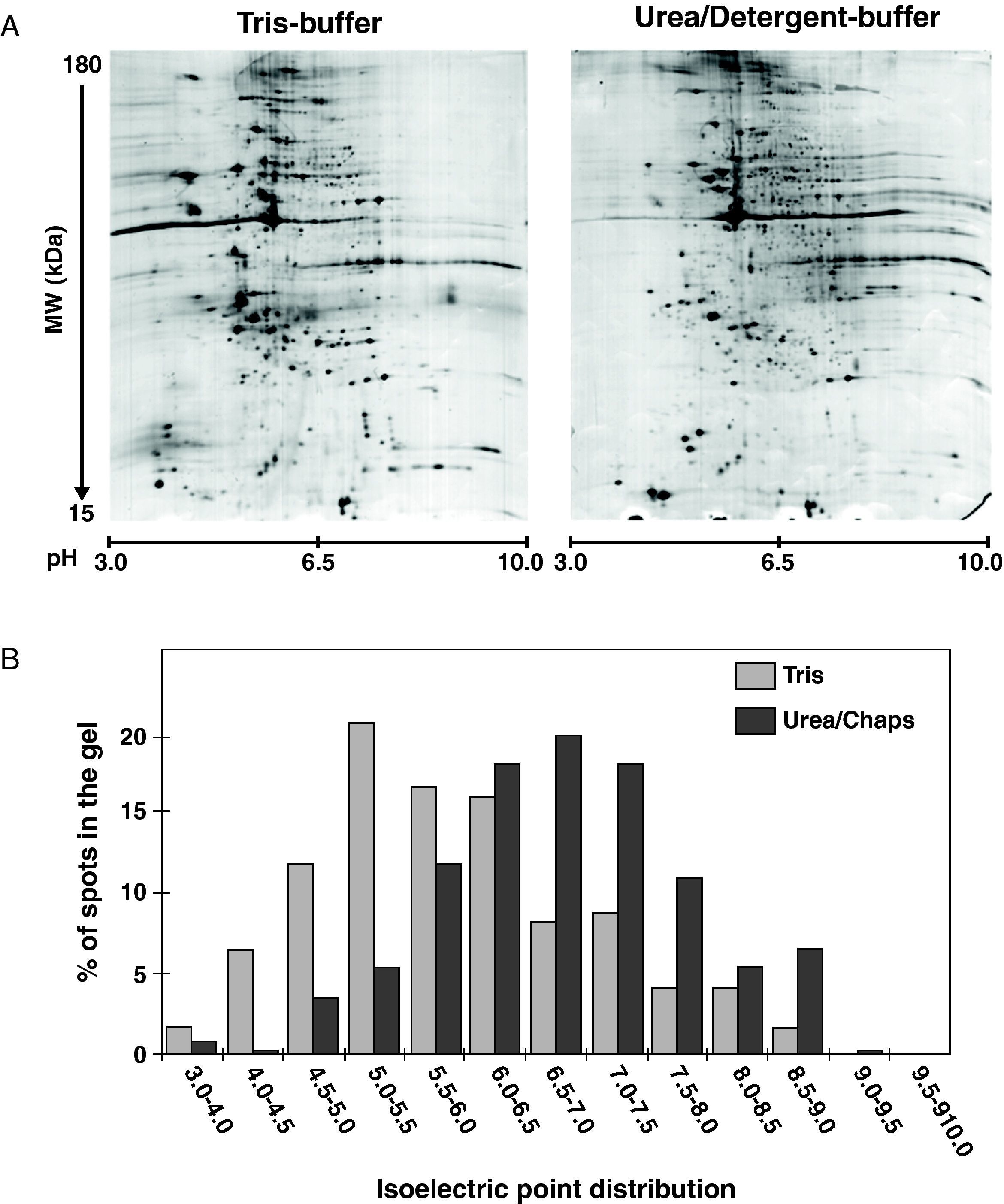
3Results3.12-DE electrophoresis, protein separation, and mappingProtein profiles of human coronary VSMC culture treated with and without LDL (100μg/mL), in the presence or absence of 10μM rosuvastatin, were resolved by 2-DE electrophoresis after sequential cell extraction by a method based on different protein solubility (Figure 1). To achieve the best results from 2-DE for statistical analysis, samples for the different groups from each sub-fraction and experiment (6 samples/fraction) were processed in parallel and run together with the EttanDaltsix device. A typical example of the 2-DE gels from the tris- and urea/detergent-soluble subproteomes are shown in Figure 2A. Average gels were obtained from at least 3 independent experiments. Flamingo labelled 2-DE gels containing 120μg of protein were able to resolve approximately 550 spots (565±180). Proteins spots merely detected in the tris- or in the urea/detergent- fractions displayed different distribution patterns within the 2-D gels. Thus, more than 80% of the proteins entirely extracted by the tris-buffer (111±16, cytosol soluble proteins) had pI values ranging from 4.0 to 7.0, whereas the majority of proteins (70-80%) that only partitioned in the urea-detergent fraction (260±77, cytoskeleton/membrane associated proteins) had pIs between 6.0 and 8.5 (Figure 2B). Protein spots from both extracted fractions were distributed long molecular masses of 15-180 kDa, although a higher percentage of the spot proteins that were only detected in the tris-fraction tended to resolve within a lower molecular mass range (40% spots in tris-fraction vs. 27% in urea/detergent-fraction were under 40 kDa).
Analysis by 2D-electrophoresis of human coronary VSMC. (A) Representative images of 2DE from the Tris-soluble and urea/detergent soluble subproteomes. Protein loading was 120μg, and gels were staining using fluorescence labelling. Calibration of Mr and pI was performed with external and internal protein markers and using the PDQuest software (B) Protein distribution according the experimental pI. Note that tris-soluble proteins distributed in a more acid pH interval than the urea/detergent soluble proteins.
A 39% of the protein spots resolved by 2D-gel electrophoresis of the urea/detergent soluble fraction were found under all the studied conditions (VSMC treated with/without LDL, +/- rosuvastatin). These spots were picked, digested, and identified by MALDI-TOF mass spectrometry. Using the ProFound and Mascot search engines, we have consistently identified 164 protein spots at least in three independent gels. All identified proteins had an expectation value of 0.01 or better with the ProFound and scores over 75 with the Mascot, which ensures that risk of random identification is below the accepted limit (p<0.05). The spots identified referred to 84 non-redundant protein species (from different gene products) often represented by more than one spot, being the differences in their molecular mass Mr and/or pI probably due to the presence of post-translational modifications. Analysis of the identified proteins by the Gene Ontology (GO) tool reported that 35 proteins (42%) were located at the cell membrane, 12 of them containing an extracellular region and 6 classified as cell surface proteins. In all the gels, the most abundant spots were identified as the cytoskeleton-protein beta-actin, the predominant form of actin in cultured VSMC (23 different spots, 39±8% of the total intensity of the valid spots in the gels). In addition, 13 further identified proteins had a structural or cytoskeleton-associated activity.
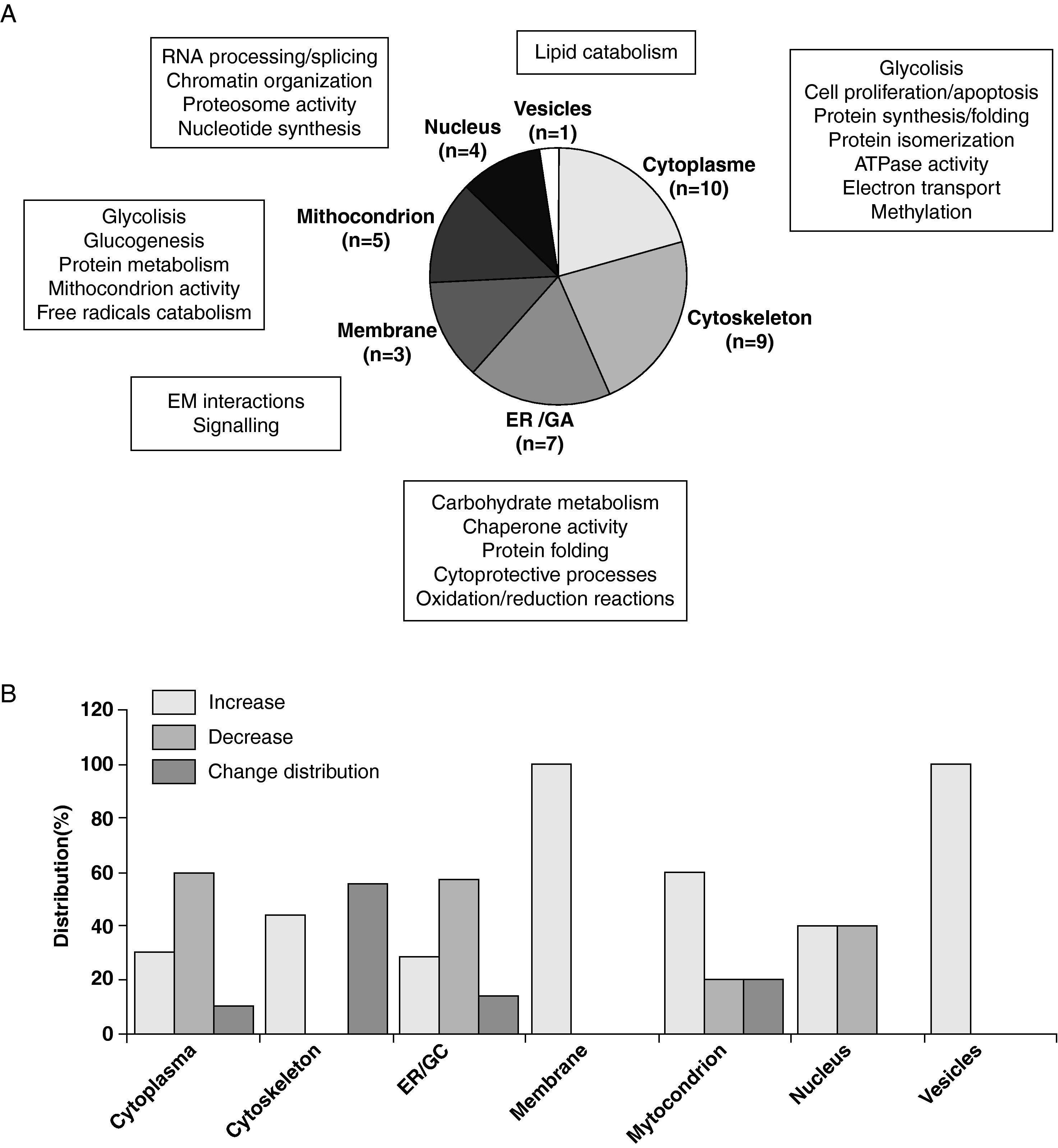
3.3Differential Proteome in LDL-loaded VSMCThe analysis of proteins differentially expressed in LDL-treated VSMC and controls revealed that 52 out of the total 164 protein spots identified in the 2DE-gels of the urea/detergent fraction showed differences of 1.5fold (defined threshold) or more in their intensity (32 upregulated- and 20 downregulated-spots). These differentially expressed spots referred to 39 non-redundant proteins. More specifically, we found 18 proteins increasing and 14 proteins decreasing their detection levels between LDL-treated and non-treated VSMC. In addition, 8 multispot proteins showed a differential spot density distribution. Application of the Ingenuity Pathways Analysis software (IPA, Ingenuity Systems) revealed that 5 biological functions (cellular motility, cell viability, cell morphology, protein and carbohydrate metabolism) were mainly represented in the differentially expressed proteins. In comparison to control VSMC, LDL-treated VSMC showed major changes in proteins involved in canonical pathways related to actin cytoskeleton signalling and regulation of the actin-based motility, oxidative stress response, glutathione metabolism, amino acid biosynthesis and protein metabolism (p<0.003 for all pathways; Figure 3).
(A) Pie chart representing the subcellular distribution and biological functions of the 39 differential proteins identified in LDL-treated human coronary VSMC compared to control cells. Assignments were made on the basis of information provided on the Swiss-Prot web-site and using the Gene Ontology (GO) and the Ingenuity Pathways Analysis (IPA) tools. (B) Bar diagrams denote the percentage of proteins increasing (>1.5fold), decreasing (>1.5fold), or shifting their proteomic pattern (multispot proteins) in the different cellular organelles and compartments of LDL-treated VSMC compared to control cells.
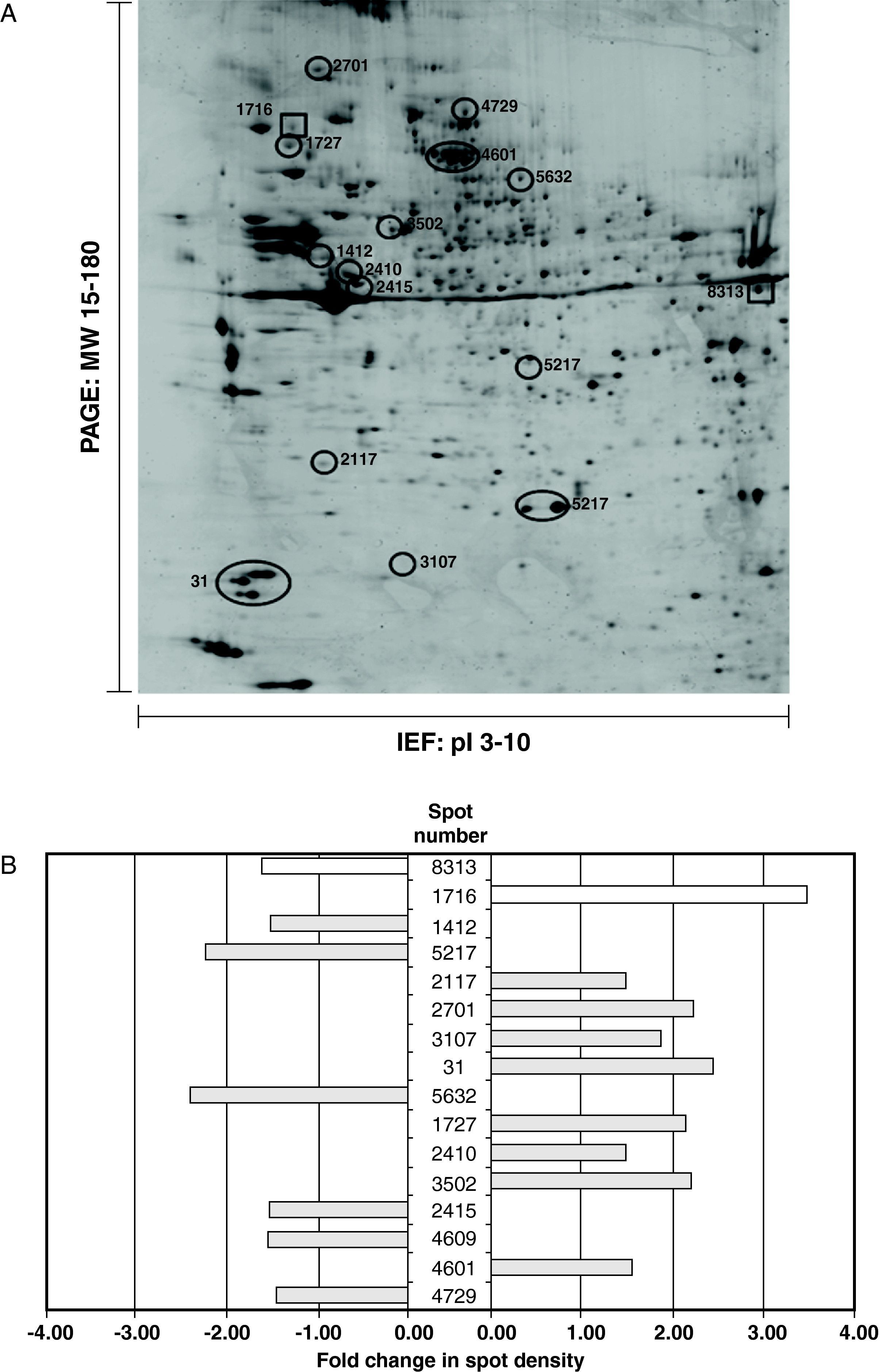
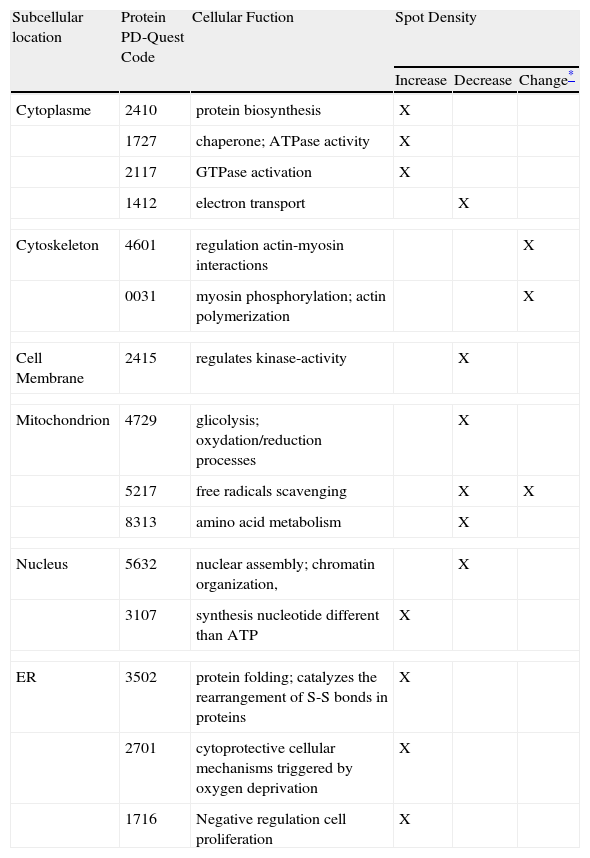
Human coronary VSMC were treated with/without 100μg/mL LDL in the presence or absence of 10uM rosuvastatin for a period of 24hours. Proteomic analysis from 3 independent experiments revealed that rosuvastatin suppressed or induced 1.5fold or more the expression level of 42 of the 164 protein spots (29%) identified in the 2DE-gels of the urea/detergent fraction (26 proteins increased 1.5-8.0fold, 16 proteins decreased 1.5-5.0fold) in the LDL-treated group. Thus, rosuvastatin normalized the density values of 13 proteins out of the 39 non-redundant proteins with differential proteomic pattern in the LDL-treated VSMC, (4 decreased, 6 increased, 3 multispot proteins changed their distribution), whereas intensity levels of 2 proteins were not only completely reverted by rosuvastatin but significantly increased/decreased in comparison to controls (Figure 4). The remaining 24 proteins were not consistently affected by the presence of rosuvatatin. In the LDL-group, rosuvastatin affected the proteomic profile of 19 further proteins (13 upregulated, 6 downregulated) that otherwise were not modified by the presence of LDL.
(A) Representative 2D-electrophoresis gel image of the urea/detergent soluble fraction in LDL-VSMC treated with 10μM rosuvastatin. The 13 circled spots refer to the proteins with changes in their density of 1.5fold or more vs the LDL-VSMC group and that have a normalized density pattern when compared with control cells. The spots marked with box refer to those proteins with density values reversed by the effect of rosuvastain and >1.5 increased/decreased compared to the control group. (B) Histogram of protein abundance ratio obtained by the PDQuest software LDL-VSMC treated with or without rosuvastatin.
Proteins of the LDL-VSMC that normalized their proteomic profile by the presence of rosuvastatin were components of the cell membrane (1 protein), endoplamatic reticulum (2 proteins), nucleus or nuclear lamina (2 proteins), mitochondrion (2 proteins), cytoskeleton (2 proteins), and cytoplasmatic compartment (4 proteins), According to GO, IPA, and PubMed reported information, rosuvastatin affected different functional groups of proteins in the LDL-VSMC group, including proteins directly involved in the assembly/disassembly, cross-linking and stabilization of the actin cytoskeleton network, proteins with chaperone and ATPase activity, trafficking proteins, metabolism-associated proteins, and proteins involved in cell cycle and viability (Table 1). The 19 proteins affected by rosuvastatin independently of LDL presence were, according to the GO notations, mainly involved in cytoskeleton interactions and cytoskeleton dynamics (5 proteins), regulation of protein folding (4 proteins), and regulation cell redox (2 proteins). Other functions were related to proteosome activity, glycolisis, methylation processes, formation ion channels, and protein metabolism.
Group of proteins with differential expression between rosuvastatin-treated and non-treated LDL-VSMC.
| Subcellular location | Protein PD-Quest Code | Cellular Fuction | Spot Density | ||
| Increase | Decrease | Change* | |||
| Cytoplasme | 2410 | protein biosynthesis | X | ||
| 1727 | chaperone; ATPase activity | X | |||
| 2117 | GTPase activation | X | |||
| 1412 | electron transport | X | |||
| Cytoskeleton | 4601 | regulation actin-myosin interactions | X | ||
| 0031 | myosin phosphorylation; actin polymerization | X | |||
| Cell Membrane | 2415 | regulates kinase-activity | X | ||
| Mitochondrion | 4729 | glicolysis; oxydation/reduction processes | X | ||
| 5217 | free radicals scavenging | X | X | ||
| 8313 | amino acid metabolism | X | |||
| Nucleus | 5632 | nuclear assembly; chromatin organization, | X | ||
| 3107 | synthesis nucleotide different than ATP | X | |||
| ER | 3502 | protein folding; catalyzes the rearrangement of S-S bonds in proteins | X | ||
| 2701 | cytoprotective cellular mechanisms triggered by oxygen deprivation | X | |||
| 1716 | Negative regulation cell proliferation | X | |||
Changes induced by rosuvastatin in 10 non-redundant proteins (6 downregulated, 4 upregulated) were detected with a similar pattern in the control group. Additionally rosuvastatin modified detection levels of 4 proteins (2 downregulated, 2 upregulated) in the control- but not in the LDL-treated group.
4DiscussionThe aim of our study was to unravel the effect of rosuvastatin on the proteome of VSMC in response to atherogenic levels of LDL. Proteins play an important role in regulating all biological systems in response to cellular stress and pathological triggers. Consequently the study of the proteome may provide clues for new targets and molecular pathways involved in the effects of the rosuvastatin modulating the VSMC response to an atherogenic environment.
A 2D-electrophoresis/MALDI-TOF MS approach was used for the separation and identification of differentially expressed proteins in cellular subsets of VSMC. Our results showed a high level of complexity of the VSMC proteome in response to LDL.
Among the 84 non-redundant proteins unambiguously identified by MS in the urea/detergent soluble fraction of human coronary VSMC, 39 proteins showed significant changes (increase/decrease) in their abundance or changes in the proteomic pattern. Indeed, some of the proteins were identified in several spots, corresponding to different isoforms, suggesting that posttranslational modifications are common. Posttranslational modifications are normally associated with protein activity. The fact that only specific isoforms from the same protein presented a changing behaviour, with increased or decreased expression levels in LDL-treated compare to control VSMC could reflect the differential activation/inactivation stage of those proteins associated to the presence of LDL.
Our study has evidenced that rosuvastatin reversed to control levels the 30% of LDL-induced effects in human coronary VSMC. Previous studies have reported that statins modulate the levels of proteins secreted by cultured atherosclerotic plaques22 and modify the protein profile of circulating human monocytes after an acute coronary syndrome23. To our knowledge, however, the effect of statins on the proteomic response of VSMC to atherogenic LDL has not been previously addressed.
The protective effects of rosuvastatin refer to proteins involved in different biological functions and located in various subcellular organelles and compartments. Thus, 2 of the proteins normalized by rosuvastatin were found to be involved in protein biosynthesis and folding. Newly synthesized proteins form disulfide bonds and gain their three-dimensional structure in the ER. An inadequate protein folding derives in the accumulation of unfolded proteins inducing ER stress24. To this respect normalization of the protein folding decreases ER-derived oxidative stress25 and consequently represses activation of signal transduction system called the unfolded protein response (UPR)26,27. The UPR is initially an adaptive response but, if unresolved, can lead to apoptotic VSMC death24. Thus, therapeutic interventions that reduce ER stress seem to be promising strategies to treat cardiovascular diseases.
A large number of proteins involved in cellular defence mechanisms are related to cellular stress. Rosuvastatin reversed LDL effects on proteins involved in free radical scavenging, the most evident changes were found in proteins located in the mitochondrion, which support a statin-mediated decrease of mitochondrial ROS-production, ER-stress and UPR activation25.
Rosuvastatin counteracts the effects of LDL on different proteins associated to the actin-cytoskeleton. We have recently demonstrated that LDL impair vascular remodeling and repair because of changes affecting cytoskeleton proteins10,11. The finding that rosuvastatin normalized the proteome profile of actin-associated proteins relevant for cytoskeleton dynamics might have a favourable effect in cell motility which in turn could relate with the plaque stabilization effects attributed to the statins16.
Proteins facilitating nuclear assembly and chromatin organization were decreased and those negatively regulating cell proliferation were increased by the presence of rosuvastatin. On line with these results, previous studies have demonstrated that statins attenuated proliferation of cultured smooth muscle cells by interfering with G-protein-mediated cell cycle regulation28.
In summary, by analyzing the effect of rosuvastatin on the proteomic profile of lipid-rich human coronary VSMC we have identified candidate proteins involved in cellular processes known to be relevant in atherosclerosis. Therefore, these proteins may provide new clues in the protective effect of rosuvastatin in cardiovascular pathology.
DisclosuresNo conflicts to disclose.
This work has been possible due to a grant of the Spanish Society of Atherosclerosis (Beca ASTRAZENECA 2005). The work has been also supported by funds provided by SAF 2006/10091 to L.B., CIBER OBENU CB06/03 to L.B., FIS-PI071070 to T.P., and “Fundación Jesus Serra”.
The work was partially presented as an oral communication entitled, “Efecto de las LDL en el perfil proteómico de proteínas involucradas en proliferación en células musculares lisas de arterias coronarias humanas” (T. Padró, M García-Arguinzonis, L. Badimon) during the “XIX Congreso Nacional de la SEA” in Santander, 2006 and was awarded with a “Mención Especial”
The work was partially presented as an oral communication entitled, “Efecto de las LDL en el perfil proteómico de proteínas involucradas en proliferación en células musculares lisas de arterias coronarias humanas” (T. Padró, M García-Arguinzonis, L. Badimon) during the “XIX Congreso Nacional de la SEA” in Santander, 2006 and was awarded with a “Mención Especial”.