Post-prandial lipaemia (PL), oxidative stress (OS), and complement component C3 (C3) values are related to the atherosclerosis process. The post-prandial response of C3 after an oral fat load test (OFLT) using unsaturated fat is poorly addressed. The aim of this study was to analyze and compare the post-prandial response of OS markers and C3 values in men and women after an OFLT using unsaturated fat.
MethodsThe study included a total of 22 healthy subjects with normal lipids and normal blood glucose (11 men and 11 pre-menopausal women). An oral unsaturated fat load test (OFLT: 50g fat per m2 body surface) was performed using a commercial liquid preparation of long chain triglycerides (Supracal®). OS markers and C3 were measured using standardized methods at fasting state and every 2h up to 8h after the OFLT.
ResultsMen showed statistically significant higher C3, oxidized glutathione (GSSG), and oxidized-reduced glutathione (GSSG/GSH) ratio values at fasting state compared to that obtained in women. In addition, post-prandial C3 values and GSSG/GSH ratios were significantly higher in men compared to women. The GSSG value and GSSG/GSH ratio significantly decreased in men after the OFLT compared to fasting values. In contrast, the post-prandial OS markers decrease observed in women was not statistically significant.
ConclusionsIn fasting state, men showed higher statistically significant C3 values and OS markers than women. The post-prandial OS markers (GSSG and GSSG/GSH ratio) significantly decrease after the OFLT with unsaturated fat in men compared to women.
Los valores de lipemia postprandial (PL), estrés oxidativo (OS) y componente C3 del complemento (C3) están relacionados con el proceso de aterosclerosis. La respuesta postprandial de C3 tras una sobrecarga oral de grasa (OFLT) utilizando grasa insaturada no es completamente conocida. Nuestro objetivo fue analizar y comparar la respuesta postprandial de los marcadores de OS y los valores de C3 en hombres y mujeres después de una OFLT utilizando grasa insaturada.
MétodosEstudiamos 22 sujetos normolipidémicos y normoglicémicos (11 hombres y 11 mujeres premenopáusicas). Se realizó una sobrecarga oral con grasa insaturada (OFLT: 50g de grasa por m2 de superficie corporal) utilizando una preparación líquida comercial de triglicéridos de cadena larga (Supracal®). Los marcadores OS y C3 se midieron utilizando métodos estandarizados en estado de ayuno y cada 2 horas hasta 8 horas después de OFLT.
ResultadosLos hombres mostraron valores significativamente mayores de C3, glutatión oxidado (GSSG) y glutatión reducido (GSSG/GSH) en estado de ayuno en comparación con los obtenidos en mujeres. Además, los valores de C3 postprandiales y la relación GSSG/GSH fueron significativamente más altos en los hombres que en las mujeres. El valor GSSG y la relación GSSG/GSH disminuyeron significativamente en los hombres después de OFLT en comparación con los valores de ayuno. En contraste, la disminución de marcadores postprandiales de OS observada en mujeres no fue estadísticamente significativa.
ConclusionesEn ayunas, los hombres muestran valores estadísticamente mayores de C3 y marcadores OS que las mujeres. Los marcadores OS postprandial (GSSG y GSSG/GSH ratio) disminuyen significativamente tras OFLT con grasa insaturada en los hombres en comparación con las mujeres.
In the last decade dietary habits have changed and the number of meals has increased, thus the majority of individuals spend most of each day in a postprandial state. Postprandial lipemia (PL) is determined by the accumulation in plasma of triglyceride-rich lipoproteins (TRL) such as chylomicrons (QM), lipoproteins of very low density (VLDL) and both particles between 6 and 10h after a meal.1 It has been suggested that 40% of all patients with premature coronary artery disease have normal fasting plasma lipids, but impaired clearance of PL lipoproteins.2 In addition, this postprandial impaired clearance of lipoproteins appears to be exaggerated in subjects with abdominal obesity, diabetes and metabolic syndrome, all conditions with high cardiovascular risk.1,2
Oxidative stress (OS), an excessive production of reactive oxygen species (ROS) not compensated by the mechanisms of antioxidant defense, has been associated with cellular events, such as inactivation of nitrate oxidase, oxidative modifications of DNA and proteins, lipid oxidation, increase of the mitogenicity and apoptosis of the vascular cells that contribute to the development and progression of atherosclerosis. The OS can be modulated by factors such as age, gender and nutritional status.2,3
In the postprandial state, lipids and circulating lipoproteins can modulate the OS.2,3 Diet interventions, test meals and oral fat load test (OFLT) in healthy subjects have shown that the type of fat may regulate postprandial OS.1,2 After the consumption of a meal high in dietary energy, typically rich in carbohydrates and/or saturated fats, ROS production increases in relation to the degree of hyperglycemia and/or hyperlipidemia.3 Beneficial effects have been shown when unsaturated fat was used compared to the saturated fat.1,2 In this sense, a high saturated fat meal induced endothelial dysfunction and was associated with higher degree of OS.4 In another study, Wallace et al. concluded that OS generated by altered PL can damage endothelial function.2 In studies conducted with test meals both postprandial hypertriglyceridemia and hyperglycemia had adverse effects on endothelial function and inflammatory markers, mediated by the OS.3,5
Up to date, few studies have compared postprandial OS and antioxidant capacity between men and women.6–9 Some studies have focused on exercise-induced OS rather than on resting levels, and most studies have been conducted using older populations, often involving postmenopausal women and age-matched men, in an attempt to investigate the potential influence of estrogen on OS.
Chronic low-grade inflammation, as OS, is an essential element in the pathogenesis of atherosclerosis. In the process of inflammation, we can include the complement system, a key component of innate immunity and necessary in responses mediated by antibodies. The three-way activation of the complement system converge in C3 (the most abundant protein of the complement in blood), resulting in the release of anaphylatoxins (C3a, C4a, C5a) that stimulate mast cells to release histamine and proteases that contribute to vascular alterations in the pathogenesis of atherosclerosis.10
Fasting complement component C3 (C3) has been associated with metabolic syndrome, abdominal obesity, insulin resistance and acute myocardial infarction. In addition, postprandial C3 predicted insulin resistance and was higher in men with familial combined hyperlipidemia compared to women.11,12 Moreover, postprandial C3 plasma concentrations increased in patients with coronary artery disease and decreased with statin treatment.13
Regarding the effect of the gender in the PL, women have an area under the curve (AUC) of triglyceridemia lower compared to men, being the largest difference between women and men with metabolic syndrome.6 In addition, men with metabolic syndrome have a more delayed clearance of postprandial triglyceridemia compared to women.14 In contrast, some data indicate that estradiol concentrations had no significant effect on postprandial triglycerides or biomarkers of OS or inflammation in a sample of young and healthy women.9 The study conducted by Couillard et al. suggested that the gender effect on PL is due to the accumulation of visceral adipose tissue.15
Our research group demonstrated in a healthy population, a greater AUC of diurnal capillary TG (TGc) in men, postmenopausal women and women with an abdominal obesity compared to healthy women. The AUC of diurnal TGc mayor predictors were age, gender and BMI.16,17 Recently, we have demonstrated that hypercholesterolemic patients have higher fasting OS markers and that an OFLT with predominantly unsaturated fat decrease postprandial OS status in hypercholesterolemic patients.18 However, this beneficial effect was higher than that obtained in normolipidemic normoglucemic subjects.19 These results probably indicate that those subjects with higher fasting OS status will obtain a better postprandial response using unsaturated fat.
In summary, it is well known that PL, C3 values, OS and inflammatory markers related to the atherosclerosis process are higher in healthy men compared to women. Unsaturated fat has demonstrated a decrease in postprandial TG, OS and inflammatory markers in different populations. The postprandial response of C3 after an OFLT using unsaturated fat is poorly addressed. Therefore, the aim of our study is to analyze and compare postprandial response of OS markers and C3 values in men and women after an OFLT using unsaturated fat. Our hypothesis is that postprandial OS markers and C3 values after an OFLT could be modulated by gender.
Subjects and methodsSubjectsWe have studied 22 healthy subjects (11 men and 11 premenopausal women). All the subjects were normoglycemic (fasting serum glucose<100mg/dl) and normolipidemic (fasting TC<200mg/dl and fasting TG<150mg/dl). The subjects were randomly selected from plasma donors, researchers and staffs at our Center.
The inclusion criteria were: total cholesterol (TC)<200mg/dl, TG<150mg/dl, apolipoprotein B<120mg/dl, fasting glycemia<100mg/dl, and absence of personal or family history of dyslipidemia, cardiovascular disease and diabetes. A body mass index (BMI) less than 30kg/m2, age between 18 and 45 years and E3/E3 genotype of apoprotein E were demanded.
The exclusion criteria were: weight variations greater than 10% of total body weight over the past 3 months, intake of drugs capable of modifying lipid profile, intake of vitamins or antioxidant supplements, low calorie diet, intake of more than 30galcohol/day, diabetes, gravity or breast feeding, metastatic neoplastic disease, liver cirrhosis, thyrotropin greater than 10mU/ml, creatinine greater than 2mg/dl, cholestasis (presence of 3 different criteria: GGT>32mU/ml, bilirubin>0.2mg/dl and alkaline phosphatase>250mU/ml), postmenopausal state, personal history of amenorrhea or polycystic ovary syndrome, more than 4h per week of exercise and smokers.
The study was approved by the Ethics Committee of our Centre and the subjects gave their writing consent to participate in the study.
Clinical and anthropometric parametersIn the study protocol, among others, the following clinical parameters were recorded: smoking, consumption of alcohol (grams of alcohol per day), physical exercise (hours/week) and use of regular or occasional drugs and vitamin or antioxidant supplements that match with the date of the study.
Blood pressure was determined using a standardized procedure (Mercury sphygmomanometer). The anthropometric parameters were collected using standardized procedures: weight (kg), height (m), BMI (kg/m2) and the waist circumference (midpoint between the edge lower rib and iliac crest, in centimeters). All these measurements were done by the same researcher.
Oral fat load testSubjects ingested a high-fat meal with a commercial liquid preparation of long chain triglycerides (Supracal®; SHS International Ltd). Each 100ml contains 50g of fat (450 Kcal), of which 9.6g are saturated, 28.2g are monounsaturated and 10g are polyunsaturated. The ratio ω6/ω3 is>20/1. The dose ingested was 50g fat per m2 body surface.
The study started at 8:30am, after a 12h overnight fast. Subjects rested for 30min before the first blood sample extraction. After that, the liquid preparation of lipids (Supracal®) was administered. The participants remained sitting or supine during the next 8h and were only allowed to drink mineral water. Peripheral blood samples were obtained in sodium EDTA before (t 0) and at regular time intervals of 2h up to 8h after the OFLT.
Laboratory methodsMeasurement of lipids and lipoproteinsAfter 12h fast, blood samples were drawn from an antecubital vein in tubes containing EDTA (Vacutainer) and were centrifuged within 4h. Plasma was stored at 4°C for a maximum of 3 days. Cholesterol and triglyceride levels were measured by standard enzymatic techniques. High density lipoprotein cholesterol (HDL-C) was measured after precipitation of apoB-containing lipoproteins with polyanions and very low density lipoprotein cholesterol (VLDL-C) after separation of VLDL (d<1.006g/ml) by ultracentrifugation. The LDL-C was calculated by subtraction of VLDL and HDL cholesterol from total cholesterol. Total plasma apoB was measured by immunoturbimetry. The coefficients of variation for lipids and lipoproteins were<5%. Fasting glucose was determined using enzymatic methods and fasting insulinemia values using a standardized ELISA for calculation Homeostasis Model Assessment- Insulin resistance index (HOMA-IR). C3 plasma values were measured by immunonephelometry. All procedures were standard and have been previously published.19
Oxidative stress assaysMarkers of OS were determined in circulating linfo-monocytes isolated by Ficoll-Hypaque methods as previously reported.18 Oxidized and reduced glutathione (GSSG and GSH) were analyzed by high performance liquid columns (HPLC) and UV detection.19
Statistical analysisData were analyzed using the Statistical Package for the Social Sciences (SPSS 12.1.3 for Windows; SPSS Chicago, IL, USA). For each variable, values are given as mean±SD. Sample size was determined for a desired p value of 0.05 and 80% power to detect a difference of more than 40% in OS variables between men and women. A sample size of a minimum of 10 per group, matched by age, was considered satisfactory because postprandial situation should duplicate the differences expected on the fasting state.
The area under the curve (AUC) and the differential of the AUC were calculated using Graph Pad Software (5.01 for windows; La Jolla, CA, USA).
Mann–Whitney test was used to assess differences in measured parameters at various time intervals after the OFLT between both groups. P values were adjusted for BMI. Wilcoxon test was used for comparison of data before and after the OFLT in the same subjects.
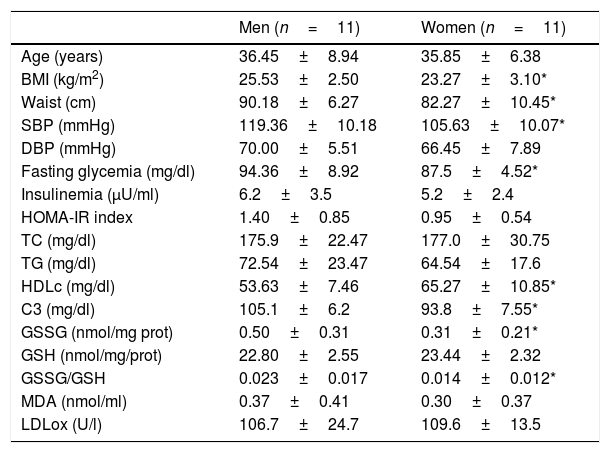
ResultsWe have studied a total of 11 healthy premenopausal women and 11 healthy men (see inclusion and exclusion criteria). The clinical and biological characteristics in the fasting state are shown in Table 1. As expected, we found statistical significant differences in BMI, waist and systolic blood pressure when compare men and women. No statistically significant differences were found in age, diastolic blood pressure, fasting TC, TG, insulinemia and HOMA-IR index between men and women. HDLc levels were significantly higher in women compared to those obtained in men.
Clinical and biological characteristics in the fasting sate of the studied subjects divided according to gender.
| Men (n=11) | Women (n=11) | |
|---|---|---|
| Age (years) | 36.45±8.94 | 35.85±6.38 |
| BMI (kg/m2) | 25.53±2.50 | 23.27±3.10* |
| Waist (cm) | 90.18±6.27 | 82.27±10.45* |
| SBP (mmHg) | 119.36±10.18 | 105.63±10.07* |
| DBP (mmHg) | 70.00±5.51 | 66.45±7.89 |
| Fasting glycemia (mg/dl) | 94.36±8.92 | 87.5±4.52* |
| Insulinemia (μU/ml) | 6.2±3.5 | 5.2±2.4 |
| HOMA-IR index | 1.40±0.85 | 0.95±0.54 |
| TC (mg/dl) | 175.9±22.47 | 177.0±30.75 |
| TG (mg/dl) | 72.54±23.47 | 64.54±17.6 |
| HDLc (mg/dl) | 53.63±7.46 | 65.27±10.85* |
| C3 (mg/dl) | 105.1±6.2 | 93.8±7.55* |
| GSSG (nmol/mg prot) | 0.50±0.31 | 0.31±0.21* |
| GSH (nmol/mg/prot) | 22.80±2.55 | 23.44±2.32 |
| GSSG/GSH | 0.023±0.017 | 0.014±0.012* |
| MDA (nmol/ml) | 0.37±0.41 | 0.30±0.37 |
| LDLox (U/l) | 106.7±24.7 | 109.6±13.5 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglycerides; HDLc, high density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment – insulin resistance; C3, complement component C3; GSSG, oxidized glutathione; GSH, reduced glutathione; MDA, malonildialdeide; LDLox, oxidized LDL.
Men showed statistical significant higher values of C3, GSSG and GSSG/GSH ratio at fasting state compared to that obtained in women (105.1±6.2 vs 93.8±7.5mg/dl, 0.5±0.31 vs 0.31±0.21nmol/mg prot, 0.023±0.017 vs 0.014±0.012, p<0.05 respectively). Men's fasting GSSG and GSSG/GSH ratio doubled GSSG and GSSG/GSH ratio women's values.
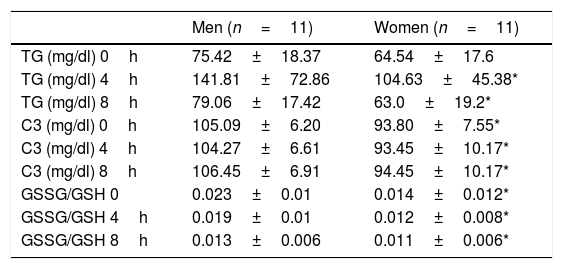
In Table 2 we show the values of TG, C3 and GSSG/GSH ratio at fasting, 4 and 8h after the OFLT. We found no statistical significant differences between men and women in the fasting TG values and postprandial TG at 4 and 8h after the OFLT. In addition, there was no statistical significant difference in the AUC of TG when comparing men versus women.
Values of triglycerides, complement component C3 and GSSG/GSH ratio in fasting, 4 and 8h after the oral fat load test with unsaturated fat.
| Men (n=11) | Women (n=11) | |
|---|---|---|
| TG (mg/dl) 0h | 75.42±18.37 | 64.54±17.6 |
| TG (mg/dl) 4h | 141.81±72.86 | 104.63±45.38* |
| TG (mg/dl) 8h | 79.06±17.42 | 63.0±19.2* |
| C3 (mg/dl) 0h | 105.09±6.20 | 93.80±7.55* |
| C3 (mg/dl) 4h | 104.27±6.61 | 93.45±10.17* |
| C3 (mg/dl) 8h | 106.45±6.91 | 94.45±10.17* |
| GSSG/GSH 0 | 0.023±0.01 | 0.014±0.012* |
| GSSG/GSH 4h | 0.019±0.01 | 0.012±0.008* |
| GSSG/GSH 8h | 0.013±0.006 | 0.011±0.006* |
Abbreviations: GSSG, glutathione in oxidized state; GSH, glutathione in reduced state; TG, triglycerides; C3, complement component C3.
As shown in Table 2, when comparing men and women results, we found statistically significant differences in C3 values and GSSG/GSH ratio in fasting and at 4 and 8h after the OFLT. Postprandial C3 values and GSSG/GSH ratio were significantly higher in men compared to women.
In Table 2 we show the changes of GSH, GSSG and GSSG/GSH ration values during the OFLT. In both, men and women, we found a statistical significant increase in GSH values at 8h after the OFLT compared to fasting and 4h after the OFLT GSH values. In men, a statistical significant decrease in GSSG values was observed during the OFLT (from 0.5nmol/mg prot to 0.36 and 0.31nmol/mg prot, p=0.018, p=0.005, respectively) (Fig. 1). This statistical significant decrease was not observed in women (0.31nmol/mg prot to 0.29 and 0.29nmol/mg prot, p=0.12, p=0.32 respectively).
The GSSG/GSH ratio significantly decreased in men at 4 and 8h after the OFLT compared to fasting values (p=0.03 and p=0.005, respectively), and this decrease was not statistical significant in women.
It is interesting to note that the statistical significant differences found in GSSG values at fasting and 2h after the OFLT between groups (men vs women) disappear at 4, 6 and 8h after the OFLT (Fig. 1), due to the significant postprandial decrease observed in men. In addition, no statistically significant differences in GSH values at the different points after the OFLT were found when comparing men and women.
When comparing women and men results of the AUC of GSH and GSSG, we found no statistically significant differences (AUC of GSH 189.1±15.02 vs 195.32±13.43nmol×h/mg prot, AUC of GSSG 3.35±1.86 vs 2.32±1.36nmol×h/mg prot, respectively). The AUC of GSSG/GSH ratio was statistically significantly higher in men compared to women (0.14±0.09 vs 0.09±0.06%×h, p<0.05).
These results indicate that in fasting and postprandial state, men show higher statistical significant OS markers. Interestingly, GSSG higher significantly decrease during the OFLT with unsaturated fat in men compared to women.
DiscussionIn our study, in fasting state men showed higher lympho-monocytes GSSG and GSSG/GSH ratios than women, indicating a higher fasting OS status. In several studies, gender differences were found in OS markers at fasting state.20,21 It is accepted that women exhibit low fasting OS markers. These differences can be in part attributed to the estrogen effects on mithocondrial enzymatic pathways implicated in ROS balance.20,21 In animal studies, the activities of ROS have been shown to be differently regulated in males and females and can directly be influenced by sex hormones.21,22 Estrogens can up-regulate the expression of antioxidant enzymes by activate estrogen receptors and the MAPK and NFKB pathway.20 In addition, a complex interaction has also been described between estrogen and TNF-α, which increases ROS production and decreases the NOS.22
Ingestion of high-carbohydrate or high-fat meals often results in postprandial hyperglycaemia and/or hypertriglyceridemia. This altered postprandial state has been related to a transient impairment in endothelial function, high postprandial levels of OS and inflammatory markers related to the atherosclerosis process.2,3 In postprandial state using saturated fat men showed higher OS status. In this sense, a high saturated fat meal generated higher degree of OS and altered endothelial function.2,4 In addition, Halkes et al. have observed an increase in ROS products in healthy subjects after an OFLT and after ingestion of a mixed meal with saturated fat.23 Therefore, these results indicate that a saturated fat load test leads to an increase postprandial OS status and can in part explain gender differences in the atherosclerosis process.
Our results indicate that after the OFLT with unsaturated fat (58% oleic and 26% linoleic acid) there was an improvement in antioxidative status (increase in GSH) with a decrease in OS parameters (GSSG and GSSG/GSH ratio) in both study groups. The decrease in GSSG levels and GSSG/GSH ratio were significantly higher in men after the OFLT, compared to the women's group. The GSSG and GSSG/GSH ratio fasting values significantly decreased up to 50% in men after the OFLT. Thus, unsaturated fat has an important effect on postprandial OS status, especially in men. In our study men show significant higher BMI and waist circumference and based on the established relationship between OS and abdominal fat deposits6,9,17 our observed results in men are highlighted.
Other dietary intervention studies have demonstrated that enriched meals with unsaturated fat improved fasting OS status. Cortés et al.24 using test meals with or without 25ml of olive oil or 40g of walnuts, studied the effect on OS parameters in hypercholesterolemic subjects and controls. In both groups, meals enriched with mono- or polyunsaturated fat, significantly decrease oxidized LDL particles. Moreover, Fuentes et al. demonstrated, after four weeks of dietary intervention with a diet enriched in monounsaturated fat, higher plasma nitric oxide values compared to a diet enriched in saturated fat or adding α-linolenic acid.25
This beneficial response can be attributed to the antioxidative stress effect of unsaturated fat and/or antioxidant components of olive oil or walnuts used in the mentioned studies. In our study, the antioxidant effect is directly obtained with the unsaturated fat content of Supracal®. It is free of antioxidant components or polyfenols in contrast to olive oil or walnuts, the fat used in the above mentioned studies.
On the other hand, some studies have shown that in men fasting and postprandial C3 are higher than in women. Men have higher waist circumference and HOMA-IR indexes compared to women and some authors have attributed the differences found in C3 between sexes to the different body fat distribution and lower insulin sensitivity observed in men.11–13 In our study, we have confirmed these results, since men have fasting and postprandial C3 values significantly higher than that observed in women. No postprandial effect in C3 values were observed with the unsaturated fat and the postprandial responses were similar in men and women.
The limitations of our results are: First, the OFLT is an acute intervention so that we can’t extrapolate long term beneficial effects of unsaturated fat. Second, the used of prepared fat test is a non physiological ingestion of fat. Third, we have no determined circulating estrogen. Fourth, the OFLT was done in different menstrual cycle days in studied women.
In summary, in our study postprandial OS status significantly decreased in both sexes (decrease in GSSG and AUC of GSSG/GSH ratio and increase in GSH) using an OFLT with unsaturated fat compared to fasting OS status. Interestingly, the observed decrease in postprandial OS markers in both sexes was significantly higher in men than in women. Knowledge of the postprandial lipemia physiopathology is still scarce. We have shown that postprandial response is modulated by gender in healthy subjects. Our results can contribute to the better knowledge of postprandial lipemia and could be useful to decide personalized approach to the treatment based on the gender. However, to confirm our hypothesis several controlled postprandial interventional studies using unsaturated and saturated fat on both sexes should be done.
Conflict of interestNone.
We acknowledge financial support by grants PI15/00082, from the Carlos III Health Institute, the Spanish Ministry of Health, the Spanish Ministry of Economy and Competiveness, and the European Regional Development Fund (FEDER).
Sergio Martínez Hervás is an investigator from the ‘Juan Rodes’ programme (JR18/00051) financed by the Carlos III Health Institute and the European Regional Development Fund (FEDER).