Few studies conducted in primary care setting report about age-adjusted prevalence rates of erectile dysfunction (ED). Aims of SIMETAP-ED study were to determine crude and age-adjusted prevalence rates of ED diagnosis, to compare these rates with other similar studies, and to compare prevalence rates of cardiovascular risk factors (CVRF), cardiovascular diseases (CVD), metabolic diseases and chronic kidney disease (CKD) between populations with and without ED.
MethodsCross-sectional observational study conducted in primary care setting. Population-based random sample: 2934 adult men. Response rate: 66%. A clinical interview was conducted to diagnose ED using a question derived from ED definition. The medical records of patients were reviewed to identify their CVRF and diseases associated with ED. The age-adjustments were standardized to Spanish population.
ResultsThe prevalence rates of metabolic diseases, CVD, CVRF, and CKD in population with ED were higher than population without ED, highlighting the CVD.
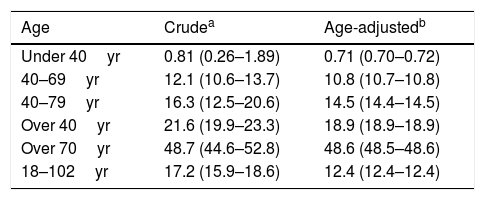
The crude prevalence of ED was 17.2% (95% confidence interval: 15.8–18.6). The age-adjusted prevalence rates of ED were 0.71% in men under 40 years, 12.4% in men over 18 years, 10.8% in men aged 40–69 years, 18.9% in men over 40 years, and 48.6% in men over 70 years.
ConclusionsSIMETAP-ED study showed association of ED with metabolic diseases, CKD, CVRF, and highlighting CVD.
The age-adjusted prevalence of ED was 12.4% in adult men, 19% in men over 40 years, and almost 50% in men over 70 years.
Existen pocos estudios realizados en atención primaria sobre prevalencias ajustadas por edad de la disfunción eréctil (ED, por sus siglas en inglés). Los objetivos del estudio SIMETAP-ED fueron determinar las prevalencias crudas y ajustadas por edad del diagnóstico de la ED, comparar estas tasas con otros estudios similares, y comparar las prevalencias de factores de riesgo cardiovasculares (FRCV), enfermedades cardiovasculares (ECV), enfermedades metabólicas y enfermedad renal crónica (ERC) entre las poblaciones con y sin ED.
MétodosEstudio observacional transversal realizado en atención primaria. Muestra aleatoria base poblacional: 2.934 varones adultos. Tasa de respuesta: 66%. Se realizó una entrevista clínica para diagnosticar ED mediante una pregunta derivada de la definición de ED. Se revisaron las historias clínicas de los pacientes para identificar sus FRCV y enfermedades asociadas con la ED. Los ajustes de tasas se estandarizaron con respecto a la población española.
ResultadosLas prevalencias de enfermedades metabólicas, ECV, FRCV y ERC en la población con ED fueron más altas que en la población sin ED, destacando las ECV.
La prevalencia cruda de la ED fue del 17,21% (intervalo de confianza del 95%: 15,86-18,63). Las tasas de prevalencia ajustadas por edad de la ED fueron del 0,71% en menores de 40 años, del 12,4% en mayores de 18 años, del 10,8% en varones entre 40 y 69 años, del 18,9% en mayores de 40 años y del 48,6% en mayores de 70 años.
ConclusionesEl estudio SIMETAP-ED mostró asociación de la ED con las enfermedades metabólicas, ERC, FRCV y, sobre todo, con ECV.
La prevalencia ajustada por edad de la ED fue del 12,4% en varones adultos, del 19% en mayores de 40 años y casi del 50% en mayores de 70 años.
The erectile dysfunction (ED) is defined as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance according to National Institutes of Health Consensus Development Panel on Impotence (NIH).1 The ED is associated with cardiovascular risk factors (CVRF), increases risk of coronary heart disease (CHD), stroke (CVA) and all-cause mortality, and may be an early manifestation of cardiovascular disease (CVD).2–4 The ED is a common disorder worldwide with higher prevalence in men over 40 years old (yr). Furthermore, ED is an important disorder in any stage of life due to unsatisfactory sex life, and psychological problems affecting quality of life and relationship with their partners.2
Many scales, single questions or self-administered questionnaires are used to diagnose ED, even though there is a main definition of ED.1 Some examples of assessment tools of ED are following: Brief Male Sexual Function Inventory (BMSFI)5 questionnaire about erectile function; Keed questionnaire,6 with 18-item for evaluation of ED, six of them for assessing erectile and orgasmic function; Boxmeer definition7 based on a positive answer on the question about problems getting an erection; Krimpen definition8 based on a questionnaire, rigidity of erections and clinical relevant. The International Index of Erectile Function (IIEF)9 is the best known. The IIEF questionnaire9 is a psychometric tool designed to assess sexual function but it has also been used as diagnostic tool for ED. It includes the erectile function domain (EF-IIEF), and the abridged 5-item version (IIEF-5),10 suitable for basic assessment work-up in patients with ED.2 Question number 15 of IIEF questionnaire9 is also used in some studies to assess confidence on getting an erection. This variability of the criteria for ED diagnoses may also increase the variability of the prevalence rates. Epidemiologic studies conducted in the same country could report different prevalence rates using different assessment tools. The variability of ED prevalence may also be explained by factors that depend on study population such as comorbidities, pharmacological treatments, ethnic groups, educational level, or socioeconomic status. Finally, the following design factors dependent on researchers may have a great influence on the variability of prevalence rates: age range of study population, type of sampling (based-population, telephone or household surveys, patients attended in medical or urologic practices), biased samples, and assessment tools (single questions, questionnaires, interviewers election surveys filled at home, by phone calling or medical consultation).
The aims of SIMETAP-ED study were to determine, in a Spanish primary care setting, the crude and age-adjusted prevalence rates of the ED diagnosis in male population aged 18 or order, to compare these rates with those of other similar studies conducted to date, and to compare the prevalence of metabolic diseases, CVRF, CVD, and chronic kidney disease (CKD) between population with and without ED.
MethodsThe SIMETAP-ED study was a cross-sectional study conducted by 121 family physicians selected from 64 primary care centers of Madrid Region Health Service (SERMAS), competitively selected until reaching the necessary sample size designed by the study. SIMETAP-ED study was included in SIMETAP study, which was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki), was approved by Research Commission of the Adjunct Management of Planning and Quality, Primary Care Management of SERMAS. All study subjects granted the informed consent. Material and methods (design, ethical aspects, sampling, recruitment, inclusion and exclusion criteria of study subjects, and data collection) were previously reported in this journal.11
For the purposes of this study, ED was considered according to the definition of the NIH Consensus Conference1 as inability to achieve or maintain an erection sufficient for satisfactory sexual performance. The diagnosis of ED was determined by the physician after asking the patient the following question derived from the NIH definition1: Do you usually have inability to achieve or maintain an erection sufficient for satisfactory sexual performance? The researchers diagnosed ED if the patients answered that they did suffer from the aforementioned inability. The definitions of the comorbidities assessed were also previously reported in this journal.11 It was considered that patients had the comorbidities assessed if these diseases or their related codes of International Classification of Primary Care – 2nd edition (ICPC-2)12 or International Classification of Diseases – 9th revision, clinical modification (ICD-9)13 were registered in their medical records.
The population attached to SERMAS was 5,144,860 adults (99.0% of census), whose health care was performed in 260 primary care centers. A population-based sample (4462 men) was obtained from all male population aged 18 and older with no upper age limit assigned to family physicians (85,871 men). From this finite population, sample size was calculated for the 95% confidence level, 0.035 for confidence interval width (margin of error 1.75%), P=0.5 for expected proportion, and considering 35% for non-responding, losses and dropouts.
Statistical analysis was performed with Statistical Package for the Social Sciences program (IBM® SPSS® Statistical release 20.0, Armonk, NY, USA). Continuous variables were analyzed with mean and standard deviation (±SD), range, median, and interquartile range Q1–Q3 (IQR). Qualitative variables, crude and age-specific prevalence estimates were calculated and presented with lower and upper limits of 95% confidence interval (CI). The Student t test or analysis of variance (ANOVA) was used for between-group comparisons for continuous variables, and the chi-square test was used for categorical variables. The multivariate logistic regression analysis with the enter method was the applied model to assess the effect on ED (dependent variable) of those CVRF and comorbidities (independent variables) that the previously performed bivariate analysis showed a statistically significant association with the dependent variable. The variables metabolic syndrome (MetS) and CVD were not included in the multivariate analysis because these entities encompass other variables already included in the analysis. All tests were considered statistically significant if two-tailed P-value was <0.05.
The prevalence rates were reported as crude and age-adjusted rates. The age-adjusted prevalence rates were calculated by the direct method,14 standardized to Spanish male adult population. The age distribution of Spanish population was obtained from the database of the Spanish Statistics Institute (INE)15 dated January 2015.
The Medline, PubMed, Embase, Google Scholar, and Web of Science databases of studies published from January 2000 to date were reviewed to compare the prevalence rates. The search strategy included the terms prevalence, erectile dysfunction, population-base, and primary care; and excluded the terms incidence, lower urinary tract symptoms, male infertility and sexual dysfunction.
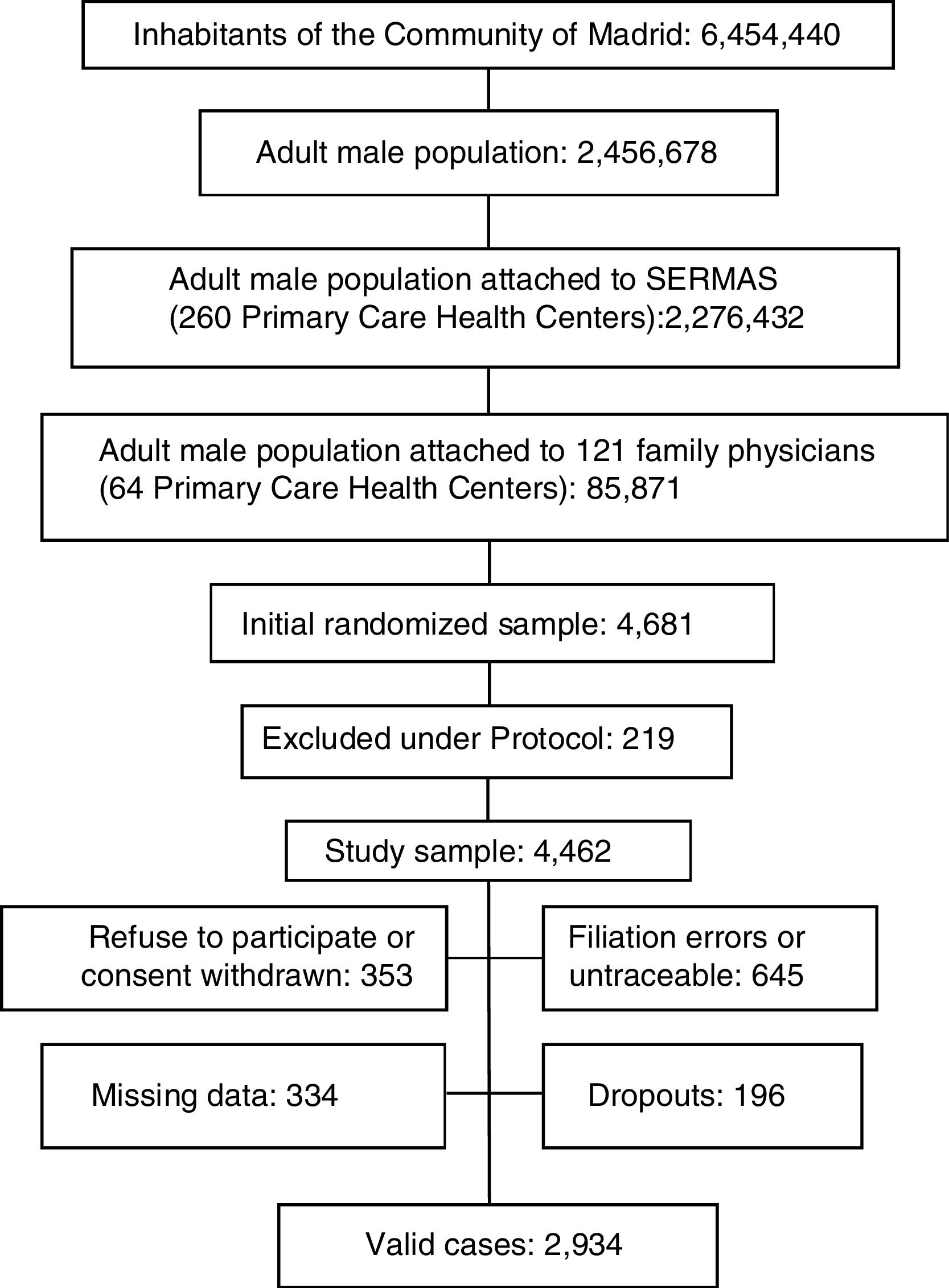
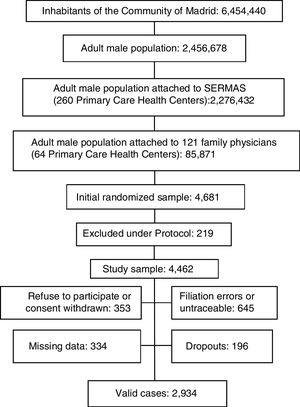
ResultsThe Spanish adult male population was 18,526,109 men and the adult male inhabitants from Madrid Region were 2,456,378 men.11 A sample with 4462 adult men from Madrid Region was obtained after excluding 4.7% per protocol (terminal patients, institutionalized patients, or with cognitive impairment). Response rate was 65.8%. Rejected participation in study or consent withdrawn was 7.6% of initial sample; 13.8% had filiation errors or were untraceable after an active search. Study subjects with missing data or with medical records without relevant clinical information were 7.1%. Losses and dropouts who did not meet the clinical interview were 4.2%. The study population was 2934 men aged 18.01–102.12yr (Fig. 1).
The median age of study population was 54.75 (IQR: 42.01–67.35) yr, and its average age was 55.06 (SD: ±16.90)yr. The range age of the population with ED was 26.06–102.12yr, and its median age was 73.28 (IQR: 62.82–81.77)yr. The range age of the population without ED was 18.01–88.73yr, and its median age was 51.26 (IQR: 39.98–63.36)yr.
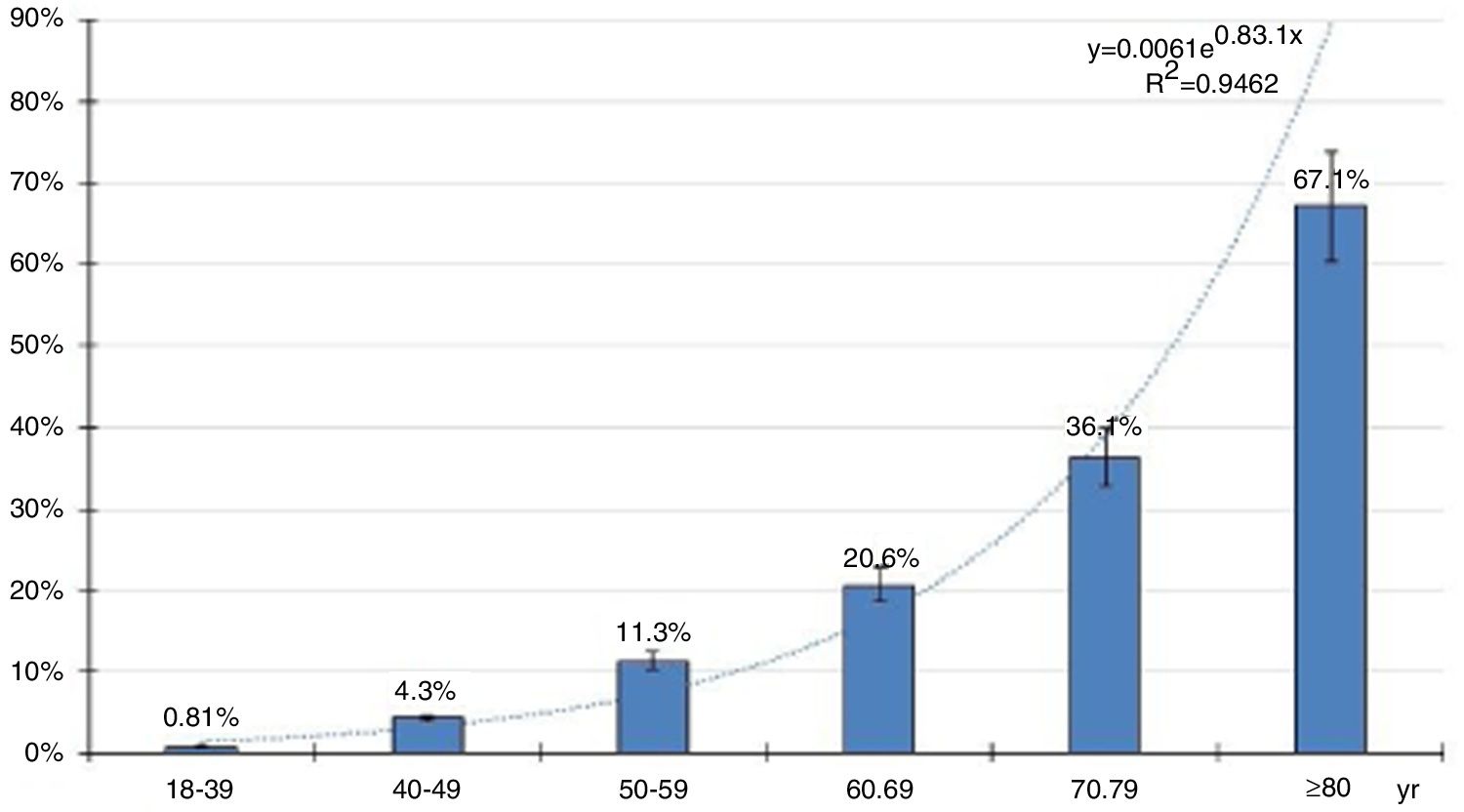
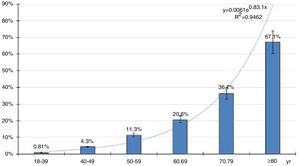
The distribution of age-specific prevalence rates of ED increases with the age according to a natural exponential function (y=0.0061e0.831x) (Fig. 2). The crude and age-adjusted prevalence rates of ED of study population are outlined in Table 1.
Prevalence rates of ED in study population.
| Age | Crudea | Age-adjustedb |
|---|---|---|
| Under 40yr | 0.81 (0.26–1.89) | 0.71 (0.70–0.72) |
| 40–69yr | 12.1 (10.6–13.7) | 10.8 (10.7–10.8) |
| 40–79yr | 16.3 (12.5–20.6) | 14.5 (14.4–14.5) |
| Over 40yr | 21.6 (19.9–23.3) | 18.9 (18.9–18.9) |
| Over 70yr | 48.7 (44.6–52.8) | 48.6 (48.5–48.6) |
| 18–102yr | 17.2 (15.9–18.6) | 12.4 (12.4–12.4) |
ED: erectile dysfunction; yr: years old.
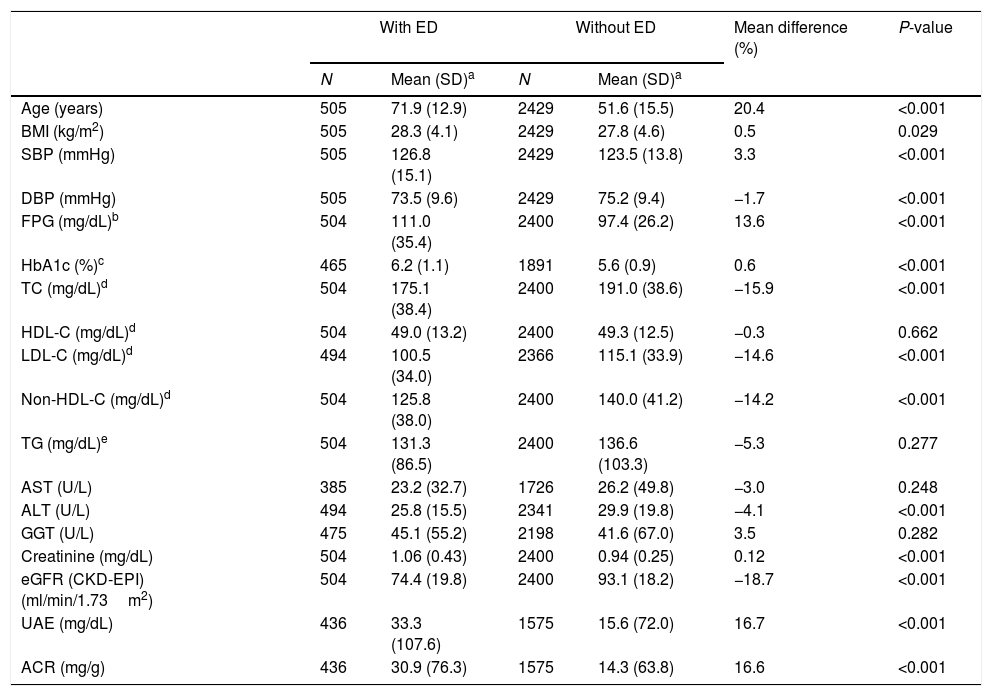
The clinical characteristics of the study population and differences between populations with and without ED are outlined in Table 2. The values of age, systolic blood pressure (SBP), fasting plasma glucose (FPG), glycated hemoglobin A1c (HbA1c), creatinine, urinary albumin excretion (UAE), and albumin-to-creatinine ratio (ACR) were significantly higher (P<0.001) in population with ED than in population without ED. The values of diastolic blood pressure (DBP), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), alanine-aminotransferase (ALT) and estimate glomerular filtration rate (eGFR) according to CKD-EPI (Chronic Kidney Disease EPIdemiology Collaboration) were significantly lower (P<0.001) in population with ED than in population without ED.
Clinical characteristics of the study population.
| With ED | Without ED | Mean difference (%) | P-value | |||
|---|---|---|---|---|---|---|
| N | Mean (SD)a | N | Mean (SD)a | |||
| Age (years) | 505 | 71.9 (12.9) | 2429 | 51.6 (15.5) | 20.4 | <0.001 |
| BMI (kg/m2) | 505 | 28.3 (4.1) | 2429 | 27.8 (4.6) | 0.5 | 0.029 |
| SBP (mmHg) | 505 | 126.8 (15.1) | 2429 | 123.5 (13.8) | 3.3 | <0.001 |
| DBP (mmHg) | 505 | 73.5 (9.6) | 2429 | 75.2 (9.4) | −1.7 | <0.001 |
| FPG (mg/dL)b | 504 | 111.0 (35.4) | 2400 | 97.4 (26.2) | 13.6 | <0.001 |
| HbA1c (%)c | 465 | 6.2 (1.1) | 1891 | 5.6 (0.9) | 0.6 | <0.001 |
| TC (mg/dL)d | 504 | 175.1 (38.4) | 2400 | 191.0 (38.6) | −15.9 | <0.001 |
| HDL-C (mg/dL)d | 504 | 49.0 (13.2) | 2400 | 49.3 (12.5) | −0.3 | 0.662 |
| LDL-C (mg/dL)d | 494 | 100.5 (34.0) | 2366 | 115.1 (33.9) | −14.6 | <0.001 |
| Non-HDL-C (mg/dL)d | 504 | 125.8 (38.0) | 2400 | 140.0 (41.2) | −14.2 | <0.001 |
| TG (mg/dL)e | 504 | 131.3 (86.5) | 2400 | 136.6 (103.3) | −5.3 | 0.277 |
| AST (U/L) | 385 | 23.2 (32.7) | 1726 | 26.2 (49.8) | −3.0 | 0.248 |
| ALT (U/L) | 494 | 25.8 (15.5) | 2341 | 29.9 (19.8) | −4.1 | <0.001 |
| GGT (U/L) | 475 | 45.1 (55.2) | 2198 | 41.6 (67.0) | 3.5 | 0.282 |
| Creatinine (mg/dL) | 504 | 1.06 (0.43) | 2400 | 0.94 (0.25) | 0.12 | <0.001 |
| eGFR (CKD-EPI) (ml/min/1.73m2) | 504 | 74.4 (19.8) | 2400 | 93.1 (18.2) | −18.7 | <0.001 |
| UAE (mg/dL) | 436 | 33.3 (107.6) | 1575 | 15.6 (72.0) | 16.7 | <0.001 |
| ACR (mg/g) | 436 | 30.9 (76.3) | 1575 | 14.3 (63.8) | 16.6 | <0.001 |
ED: erectile dysfunction; N: cases number; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin A1c; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: blood triglycerides; AST: aspartate-aminotransferase; ALT: alanine-aminotransferase; GGT: gamma-glutamyl transferase; eGFR: estimate glomerular filtration rate; UAE: urinary albumin excretion; ACR: albumin-to-creatinine ratio.
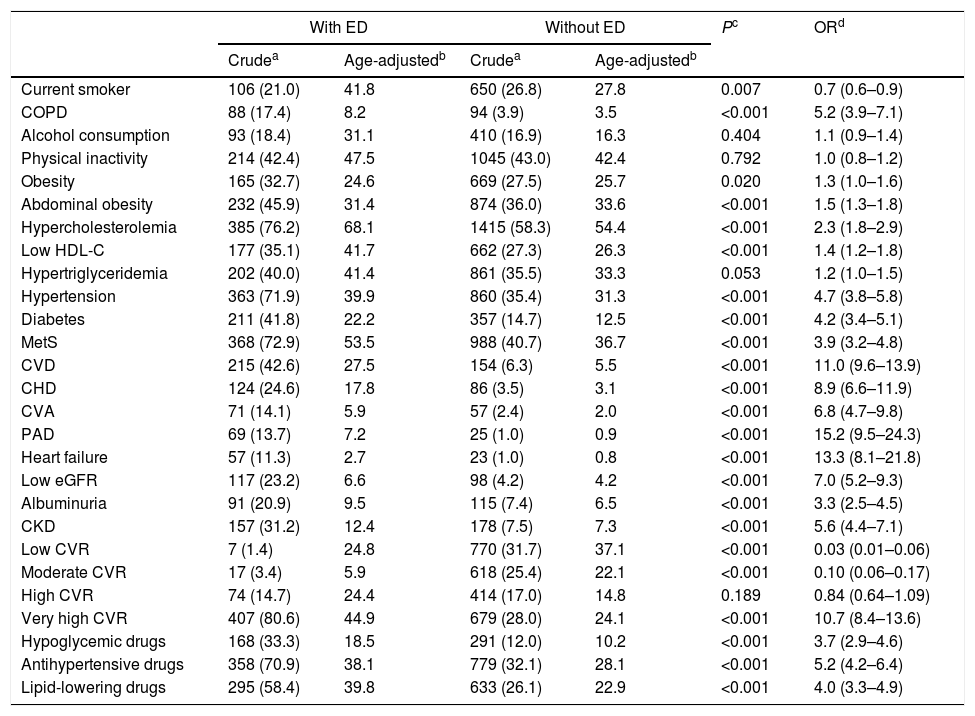
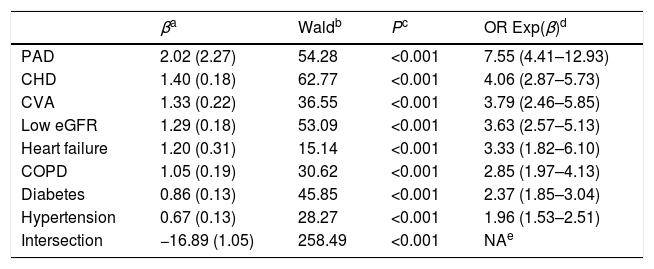
The CVRF and comorbidities of the populations with and without ED are outlined in Table 3. The differences between both populations of the percentages of all variables were significant, except alcohol consumption, physical inactivity, hypertriglyceridemia and high cardiovascular risk (CVR). The prevalence rates of the variables current smoker, low CVR and moderate CVR were significantly lower in the population with ED. The result of the multivariate analysis of the CVRF and comorbidities associated with ED is outlined in Table 4.
Comorbidity and CVRF in the populations with and without ED.
| With ED | Without ED | Pc | ORd | |||
|---|---|---|---|---|---|---|
| Crudea | Age-adjustedb | Crudea | Age-adjustedb | |||
| Current smoker | 106 (21.0) | 41.8 | 650 (26.8) | 27.8 | 0.007 | 0.7 (0.6–0.9) |
| COPD | 88 (17.4) | 8.2 | 94 (3.9) | 3.5 | <0.001 | 5.2 (3.9–7.1) |
| Alcohol consumption | 93 (18.4) | 31.1 | 410 (16.9) | 16.3 | 0.404 | 1.1 (0.9–1.4) |
| Physical inactivity | 214 (42.4) | 47.5 | 1045 (43.0) | 42.4 | 0.792 | 1.0 (0.8–1.2) |
| Obesity | 165 (32.7) | 24.6 | 669 (27.5) | 25.7 | 0.020 | 1.3 (1.0–1.6) |
| Abdominal obesity | 232 (45.9) | 31.4 | 874 (36.0) | 33.6 | <0.001 | 1.5 (1.3–1.8) |
| Hypercholesterolemia | 385 (76.2) | 68.1 | 1415 (58.3) | 54.4 | <0.001 | 2.3 (1.8–2.9) |
| Low HDL-C | 177 (35.1) | 41.7 | 662 (27.3) | 26.3 | <0.001 | 1.4 (1.2–1.8) |
| Hypertriglyceridemia | 202 (40.0) | 41.4 | 861 (35.5) | 33.3 | 0.053 | 1.2 (1.0–1.5) |
| Hypertension | 363 (71.9) | 39.9 | 860 (35.4) | 31.3 | <0.001 | 4.7 (3.8–5.8) |
| Diabetes | 211 (41.8) | 22.2 | 357 (14.7) | 12.5 | <0.001 | 4.2 (3.4–5.1) |
| MetS | 368 (72.9) | 53.5 | 988 (40.7) | 36.7 | <0.001 | 3.9 (3.2–4.8) |
| CVD | 215 (42.6) | 27.5 | 154 (6.3) | 5.5 | <0.001 | 11.0 (9.6–13.9) |
| CHD | 124 (24.6) | 17.8 | 86 (3.5) | 3.1 | <0.001 | 8.9 (6.6–11.9) |
| CVA | 71 (14.1) | 5.9 | 57 (2.4) | 2.0 | <0.001 | 6.8 (4.7–9.8) |
| PAD | 69 (13.7) | 7.2 | 25 (1.0) | 0.9 | <0.001 | 15.2 (9.5–24.3) |
| Heart failure | 57 (11.3) | 2.7 | 23 (1.0) | 0.8 | <0.001 | 13.3 (8.1–21.8) |
| Low eGFR | 117 (23.2) | 6.6 | 98 (4.2) | 4.2 | <0.001 | 7.0 (5.2–9.3) |
| Albuminuria | 91 (20.9) | 9.5 | 115 (7.4) | 6.5 | <0.001 | 3.3 (2.5–4.5) |
| CKD | 157 (31.2) | 12.4 | 178 (7.5) | 7.3 | <0.001 | 5.6 (4.4–7.1) |
| Low CVR | 7 (1.4) | 24.8 | 770 (31.7) | 37.1 | <0.001 | 0.03 (0.01–0.06) |
| Moderate CVR | 17 (3.4) | 5.9 | 618 (25.4) | 22.1 | <0.001 | 0.10 (0.06–0.17) |
| High CVR | 74 (14.7) | 24.4 | 414 (17.0) | 14.8 | 0.189 | 0.84 (0.64–1.09) |
| Very high CVR | 407 (80.6) | 44.9 | 679 (28.0) | 24.1 | <0.001 | 10.7 (8.4–13.6) |
| Hypoglycemic drugs | 168 (33.3) | 18.5 | 291 (12.0) | 10.2 | <0.001 | 3.7 (2.9–4.6) |
| Antihypertensive drugs | 358 (70.9) | 38.1 | 779 (32.1) | 28.1 | <0.001 | 5.2 (4.2–6.4) |
| Lipid-lowering drugs | 295 (58.4) | 39.8 | 633 (26.1) | 22.9 | <0.001 | 4.0 (3.3–4.9) |
ED: erectile dysfunction; CVRF: cardiovascular risk factors; COPD: chronic obstructive pulmonary disease. Alcohol consumption: more of two drinks per day. Obesity: body mass index >30kg/m2. Abdominal obesity: waist circumference >102cm. Hypercholesterolemia: total cholesterol >200mg/dL. Low HDL-C: high-density lipoprotein cholesterol <40mg/dL. Hypertriglyceridemia: triglycerides >150mg/dL. MetS: metabolic syndrome; CVD: cardiovascular disease; CHD: coronary heart disease; CVA: cerebrovascular accident or disease, stroke; PAD: peripheral arterial disease. Low eGFR: estimated glomerular filtration rate <60mL/min/1.73m2. Albuminuria: albumin to creatinine ratio >30mg/g. CKD: chronic kidney disease (low eGFR and/or albuminuria); CVR: cardiovascular risk.
Multivariate analysis of the comorbidities and CVRF associated to ED.
| βa | Waldb | Pc | OR Exp(β)d | |
|---|---|---|---|---|
| PAD | 2.02 (2.27) | 54.28 | <0.001 | 7.55 (4.41–12.93) |
| CHD | 1.40 (0.18) | 62.77 | <0.001 | 4.06 (2.87–5.73) |
| CVA | 1.33 (0.22) | 36.55 | <0.001 | 3.79 (2.46–5.85) |
| Low eGFR | 1.29 (0.18) | 53.09 | <0.001 | 3.63 (2.57–5.13) |
| Heart failure | 1.20 (0.31) | 15.14 | <0.001 | 3.33 (1.82–6.10) |
| COPD | 1.05 (0.19) | 30.62 | <0.001 | 2.85 (1.97–4.13) |
| Diabetes | 0.86 (0.13) | 45.85 | <0.001 | 2.37 (1.85–3.04) |
| Hypertension | 0.67 (0.13) | 28.27 | <0.001 | 1.96 (1.53–2.51) |
| Intersection | −16.89 (1.05) | 258.49 | <0.001 | NAe |
CVRF: cardiovascular risk factors; ED: erectile dysfunction; PAD: peripheral arterial disease; CHD: coronary heart disease; CVA: cerebrovascular accident or disease, stroke; Low eGFR: estimated glomerular filtration rate <60mL/min/1.73m2; COPD: chronic obstructive pulmonary disease.
The higher levels of FPG and HbA1c in the population with ED (Table 2) and the higher prevalence rates of treatment with hypoglycaemic drugs versus the population without ED (OR: 3.7 [CI: 2.9–4.6]) (Table 3) could be explained because the DM was strongly associated with the population with ED (OR: 4.2 [CI: 3.4–5.1]). In the multivariate analysis, the risk of developing DM in the population with ED was 2.4 (CI: 1.8–3.0) times more likely (Table 4). Likewise, the higher prevalence rates of treatment with antihypertensive drugs in the population with ED versus without ED (OR: 5.2 [4.2–6.4]) could be justified because the hypertension had a greater association with the population with ED (OR: 4.7 (CI: 3.8–5.8]) (Table 3). In the multivariate analysis, the risk of suffering from hypertension in the population with ED was 2.0 (CI: 1.5–2.5) times more likely (Table 4). Likewise, the higher prevalence rates of treatment with lipid-lowering drugs in population with ED versus without ED (OR: 4.0 [3.3–4.9]) could be justified because hypercholesterolemia and hypertriglyceridemia had a greater association with the population with ED (OR: 2.3 and 1.2 respectively) (Table 3). The lower levels of TC, LDL-C, and non-HDL-C in population with ED (Table 1) could be explained by the higher prevalence of treatment with lipid-lowering drugs in this population (Table 3). All the comorbidities outlined in Table 4 were associated with ED, highlighting the risks of suffering from peripheral arterial disease (PAD) (OR: 7.6 [CI: 4.4–12.9]), CHD (OR: 4.1 [CI: 2.9–5.7]), and CVA (3.8 [CI: 2.5–5.9]). These results support the messages that ED is considered as an independent risk factor for CVD, and that ED and CVD could be considered as manifestations of the same systemic disorder.2–4
The higher prevalence rates of MetS in population with ED versus without ED (OR: 3.9 [CI: 3.2–4.8]) could be justified because the variables abdominal obesity, hypertriglyceridemia, low HDL-C, hypertension and DM had a greater association with the population with ED (Table 2).
The higher levels of creatinine, UAE and ACR, and the lower levels of eGFR in population with ED versus without ED (Table 2) could be explained by the greater association of CKD with the population with ED (OR: 5.6 [CI: 4.4–7.1]) (Table 3). In the multivariate analysis, the risk of having low eGFR in the population with ED was 3.6 (CI: 2.6–5.1) times more likely (Table 4).
The higher prevalence rates of the very high CVR in the population with ED versus without ED (OR: 10.7 [CI: 8.4–13.6]) could be explained by the greater association of CVRF, CVD, heart failure, hypertension, DM, MetS,16 and CKD with the population with ED (Table 3).
In the other hand, the prevalence of chronic obstructive pulmonary disease (COPD) was higher in population with ED than population without ED (OR: 5.2 [3.9–7.1]) (Table 3). In multivariate analysis, the risk of suffering from COPD was 2.9 (CI: 2.0–4.1) times more likely in the population with ED, despite the fact that smoking (current smokers) was not significantly associated with ED (Table 4).
All the crude prevalence rates of the CVRF, CVD, and CKD were higher than their age-adjusted prevalence rates. This could be explained because the average and median age of the population with ED are greater than Spanish population used for the age-adjustment. Most studies agree that the prevalence of ED increases with age, and this is confirmed in the present study (Fig. 2). However, the studies included below report a high variability of prevalence rates, probably due to the different types of sampling, response rates, age ranges evaluated, and especially due to the many scales, single questions, self-administered questionnaires or criteria1,5–10 used to diagnose ED.
The crude prevalence of ED in men under 40yr was 0.8% in present study, slightly lower than crude prevalence rates reported by studies conducted in Italy17 (2%), The Netherlands18 (2.5%), Australia19 (3.5%), or Spain20 (4%).
The crude prevalence of ED in men aged 40–69yr was 12% in present study, lower than prevalence rates reported by studies conducted in Spain20 (18%), The Netherlands18 (22%), Australia19 (34%), Boston21 (35%), Brazil22 (46%), or Germany23 (59%). In this population, the age-adjusted prevalence of the present study (11%) was lower than prevalence rates reported by studies conducted in Brazil24 (16%), Italy24 (17%), Malaysia24 (22%), Japan24 (35%), Singapore25 (24%), Belgium26 (35%), New Zealand27 (38%), or Portugal28 (48%).
The age-adjusted prevalence of ED in men aged 40–79yr was 14.5% in present study, similar to prevalence rates reported by studies conducted in The Netherlands7 (13%) or Denmark29 (16%). In this population, the crude prevalence of the present study (16%) was lower than prevalence rates reported by studies conducted in Taiwan30 (18%), Germany6 (19%), Boston31 (21%), Australia32 (23%), The Netherlands18 (24%), Boston5 (25%), Jordan33 (32%), Malaysia34 (36%), or Germany23 (50%).
The crude prevalence of ED in men over 40yr with no upper limit was 22% in the present study, similar to prevalence rates reported by studies conducted in Taiwan30 (18%), Germany6 (19%), United States35 (22%), Australia32 (23%), The Netherlands18 (24%), Boston5 (25%), or Singapore25 (25%), and lower than prevalence rates reported by studies conducted in France36 (32%), Canada37 (34%), Boston21 (35%), or Malaysia34 (36%).
The prevalence of ED in men over 70yr was 49% in the present study, similar to prevalence rates of studies conducted in Italy17 (48%), or Germany6 (53%), higher than prevalence rates of Taiwan30 (34%) or The Netherlands18 (42%), and lower than prevalence rates reported by studies conducted in United States35 (55%), Australia19 (58%), or Malaysia34 (74%).
The crude prevalence of ED in men over 18yr with no upper limit was 17% in the present study, lower to prevalence rates reported by studies conducted in Singapore25 (28%), Australia19 (32%), Jordan33 (32%), Austria38 (32%), or Germany23 (35%). In this population, the age-adjusted prevalence of the present study (12.4%) was similar to prevalence rates reported by studies that also used a single question related with NIH definition1 to diagnose ED, as those conducted in The Netherlands18 (11%), Spain20 (12%), and Italy17 (13%).
Although both crude and age-adjusted prevalence rates of ED were similar in population under 40yr (0.8%) and in population over 70yr (49%), the age-adjusted prevalence of ED in entire adult male population was almost five percentage points lower than its crude prevalence, and almost three percentage points lower in men over 40yr. These differences justified the need to standardize prevalence rates due to the differences between the population structure of the sample and the Spanish population.
The main limitation of present study was that the ED degrees were not assessed. The IIEF questionnarie9 has been used to assess the efficacy of sildenafil, to identify patients at risk for ED, and to detect the severity of ED in heterosexual men with well-established partnerships. The study populations could be limited if the single men, homosexual men, divorced, and widowed were not included. On the other hand, an insufficient erection for a satisfactory sexual performance would allow to diagnose ED without the obligation to assess different degrees of erection. Others limitations included the inability of cross-sectional data to determine causation, and the possibility that men with ED did not respond or deny it.
The main strengths of present study were the population-based random selection, a large sample that included men aged 18–102yr, and the assessment of other diseases, CVRF and CVD associated with ED.
The determination of the prevalence of ED is very important to optimize the available health resources and to improve health care and quality of life for patients. The ED is strongly influenced by age, so its prevalence should always be reported with age-adjusted rates to facilitate comparison with those in other populations. Improving knowledge of the prevalence could be achieved by conducting more epidemiological studies with similar methodologies aimed at the entire population. We hope that present study will contribute to a better understanding of the ED prevalence.
ConclusionsThe SIMETAP-ED study provides the most current information about the ED prevalence in a Spanish primary care setting. The metabolic diseases, CKD, CVRF, and CVD were associated with ED, highlighting especially the CVD.
This study agrees that ED is rare in men under 40yr and that about 50% of the population over 70yr suffers from ED. The age-adjusted prevalence of ED in men over 18yr with no upper limit was 12.4%, similar to prevalence rates reported by other studies conducted in Europe. There are many studies reporting prevalence of ED in men over 40yr, however the prevalence rates are very different from each other. The age-adjusted prevalence of ED in men over 40yr was 19% in present study, and close to 11% in men aged 40–69yr. Further studies with age-adjusted rates are required to accurately determine prevalence of ED.
DeclarationsThis scientific work is original and has not been submitted or published nor is it being considered for publication in any other medium or publication.
I declare that all the authors of this scientific work agree to send it to be presented in the Journal of Clinical Research in Arteriosclerosis.
Research ethics committeeComisión de Investigación de la Gerencia Adjunta de Planificación y Calidad.
Primary Care Management. Servicio Madrileño de Salud (SERMAS).
FundingThe financing of the SIMETAP study (Grant Code: 05/2010RS) was approved according to Order 472/2010, of September 16, of the Ministry of Health, which approves the regulatory bases and the call for aid for the year 2010 of the Agency “Pedro Laín Entralgo” of Training, Research and Health Studies of the Community of Madrid, for the realization of research projects in the field of health outcomes in primary care.
Conflicts of interestThe authors have no conflicts of interest for this publication.
The assistance provided by the following physicians who have participated in the SIMETAP Study Research Group is gratefully acknowledge: Abad Schilling C, Adrián Sanz M, Aguilera Reija P, Alcaraz Bethencourt A, Alonso Roca R, Álvarez Benedicto R, Arranz Martínez E, Arribas Álvaro P, Baltuille Aller MC, Barrios Rueda E, Benito Alonso E, Berbil Bautista ML, Blanco Canseco JM, Caballero Ramírez N, Cabello Igual P, Cabrera Vélez R, Calderín Morales MP, Capitán Caldas M, Casaseca Calvo TF, Cique Herráinz JA, Ciria de Pablo C, Chao Escuer P, Dávila Blázquez G, de la Peña Antón N, de Prado Prieto L, del Villar Redondo MJ, Delgado Rodríguez S, Díez Pérez MC, Durán Tejada MR, Escamilla Guijarro N, Escrivá Ferrairó RA, Fernández Vicente T, Fernández-Pacheco Vila D, Frías Vargas MJ, García Álvarez JC, García Fernández ME, García García Alcañiz MP, García Granado MD, García Pliego RA, García Redondo MR, García Villasur MP, Gómez Díaz E, Gómez Fernández O, González Escobar P, González-Posada Delgado JA, Gutiérrez Sánchez I, Hernández Beltrán MI, Hernández de Luna MC, Hernández López RM, Hidalgo Calleja Y, Holgado Catalán MS, Hombrados Gonzalo MP, Hueso Quesada R, Ibarra Sánchez AM, Iglesias Quintana JR, Íscar Valenzuela I, Iturmendi Martínez N, Javierre Miranda AP, López Uriarte B, Lorenzo Borda MS, Luna Ramírez S, Macho del Barrio AI, Magán Tapia P, Marañón Henrich N, Mariño Suárez JE, Martín Calle MC, Martín Fernández AI, Martínez Cid de Rivera E, Martínez Irazusta J, Migueláñez Valero A, Minguela Puras ME, Montero Costa A, Mora Casado C, Morales Cobos LE, Morales Chico MR, Moreno Fernández JC, Moreno Muñoz MS, Palacios Martínez D, Pascual Val T, Pérez Fernández M, Pérez Muñoz R, Plata Barajas MT, Pleite Raposo R, Prieto Marcos M, Quintana Gómez JL, Redondo de Pedro S, Redondo Sánchez M, Reguillo Díaz J, Remón Pérez B, Revilla Pascual E, Rey López AM, Ribot Catalá C, Rico Pérez MR, Rivera Teijido M, Rodríguez Cabanillas R, Rodríguez de Cossío A, Rodríguez De Mingo E, Rodríguez Rodríguez AO, Rosillo González A, Rubio Villar M, Ruiz Díaz L, Ruiz García A, Sánchez Calso A, Sánchez Herráiz M, Sánchez Ramos MC, Sanchidrián Fernández PL, Sandín de Vega E, Sanz Pozo B, Sanz Velasco C, Sarriá Sánchez MT, Simonaggio Stancampiano P, Tello Meco I, Vargas-Machuca Cabañero C, Velazco Zumarrán JL, Vieira Pascual MC, Zafra Urango C, Zamora Gómez MM, Zarzuelo Martín N.
Research Group of SIMETAP study: Abad-Schilling C, Adrián-Sanz M, Aguilera-Reija P, Alcaraz-Bethencourt A, Alonso-Roca R, Álvarez-Benedicto R, Arranz-Martínez E, Arribas-Álvaro P, Baltuille-Aller MC, Barrios-Rueda E, Benito-Alonso E, Berbil-Bautista ML, Blanco-Canseco JM, Caballero-Ramírez N, Cabello-Igual P, Cabrera-Vélez R, Calderín-Morales MP, Capitán-Caldas M, Casaseca-Calvo TF, Cique-Herráinz JA, Ciria-de-Pablo C, Chao-Escuer P, Dávila-Blázquez G, de-la-Peña-Antón N, de-Prado-Prieto L, del-Villar-Redondo MJ, Delgado-Rodríguez S, Díez-Pérez MC, Durán-Tejada MR, Escamilla-Guijarro N, Escrivá-Ferrairó RA, Fernández-Vicente T, Fernández-Pacheco-Vila D, Frías-Vargas MJ, García-Álvarez JC, García-Fernández ME, García-García-Alcañiz MP, García-Granado MD, García-Pliego RA, García-Redondo MR, García-Villasur MP, Gómez-Díaz E, Gómez-Fernández O, González-Escobar P, González-Posada-Delgado JA, Gutiérrez-Sánchez I, Hernández-Beltrán MI, Hernández-de-Luna MC, Hernández-López RM, Hidalgo-Calleja Y, Holgado-Catalán MS, Hombrados-Gonzalo MP, Hueso-Quesada R, Ibarra-Sánchez AM, Iglesias-Quintana JR, Íscar-Valenzuela I, Iturmendi-Martínez N, Javierre-Miranda AP, López-Uriarte B, Lorenzo-Borda MS, Luna-Ramírez S, Macho-del-Barrio AI, Marañón-Henrich N, Mariño-Suárez JE, Martín-Calle MC, Martín-Fernández AI, Martínez-Cid-de-Rivera E, Martínez-Irazusta J, Migueláñez-Valero A, Minguela-Puras ME, Montero-Costa A, Mora-Casado C, Morales-Cobos LE, Morales-Chico MR, Moreno-Fernández JC, Moreno-Muñoz MS, Palacios-Martínez D, Pascual-Val T, Pérez-Fernández M, Pérez-Muñoz R, Plata-Barajas MT, Pleite-Raposo R, Prieto-Marcos M, Quintana-Gómez JL, Redondo-de-Pedro S, Redondo-Sánchez M, Reguillo-Díaz J, Remón-Pérez B, Revilla-Pascual E, Rey-López AM, Ribot-Catalá C, Rico-Pérez-MR, Rivera-Teijido M, Rodríguez-Cabanillas R, Rodríguez-de-Cossío A, Rodríguez-de-Mingo E, Rodríguez-Rodríguez AO, Rosillo-González A, Rubio-Villar M, Ruiz-Díaz L, Ruiz-García A, Sánchez-Calso A, Sánchez-Herráiz M, Sánchez-Ramos MC, Sanchidrián-Fernández PL, Sandín-de-Vega E, Sanz-Pozo B, Sanz-Velasco C, Sarriá-Sánchez MT, Simonaggio-Stancampiano P, Tello-Meco I, Vargas-Machuca-Cabañero C, Velazco-Zumarrán JL, Vieira-Pascual MC, Zafra-Urango C, Zamora-Gómez MM, Zarzuelo-Martín N.