Hyperglycerolemia is a very rare genetic disorder caused by glycerol kinase deficiency. Although usually is presented unexpectedly in routine checks, there are severe forms, especially in children. In general, glycerol and glycerol kinase activity analyses are not included in routine laboratory determination. Glycerol presents positive interferences with some biochemical analytic techniques, e.g. in serum triglycerides and plasma ethylene glycol levels assays. Here, we report a Spanish patient with a pseudo-hypertriglyceridaemia, a falsely elevated triglycerides concentration that was not corrected with lipid-lowering therapy for 3 years.
La hiperglicerolemia es una patología debida a la deficiencia de la enzima glicerol cinasa que cursa con concentraciones elevadas de glicerol. Hay diferentes manifestaciones de la enfermedad, especialmente en niños. La dificultad diagnóstica se debe a que la actividad de la enzima glicerol cinasa no está disponible en la mayoría de laboratorios públicos. Además, la cuantificación de triglicéridos en suero presenta una interferencia analítica con la determinación de glicerol ya que la mayoría de los métodos no realizan un blanco de glicerol, y este se cuantifica junto con los triglicéridos. Presentamos un caso de un niño con hiperglicerolemia de 3 años de evolución, enmascarada por una falsa elevación de triglicéridos en sangre debido a una interferencia analítica.
Hypertriglyceridaemia is a prevalent form of dyslipidemia that is frequently associated with premature coronary artery disease1 may be, due to several pathologic as a common genetic disorders of hypertriglyceridaemia, familial combined hyperlipidemia, residual dyslipemia in patients with well controlled type 2 diabetes mellitus and familial hipoalphalypoproteinemia. Hypertriglyceridaemia is characterized by increased plasma concentrations pre-β lipoproteins (VLDL) and chylomicron particles. This situation is associated with an increased risk of cardiovascular events and acute pancreatitis. Therefore, high serum triglycerides levels require an overall cardiac risk assessment. Recently, National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines identify a triglyceride of 200mg/dl (2.3mmol/l) as an important threshold for triggering “therapeutic lifestyle change”, although a normal fasting plasma triglycerides is defined as ≤150mg/dl (1.7mmol/l).2
In view of the importance of lipids to biologic and pathophysiological processes, detailed knowledge of the composition and concentration of lipid metabolites in plasma would be expected to expand our diagnostic capabilities. Here, we present a Spanish patient with a pseudo-hypertriglyceridaemia, a falsely elevated triglycerides concentration that was not corrected with lipid-lowering therapy for 3 years and whose diagnosis was determined by measuring plasma glycerol.
Case historyWe present the case of a 21-year-old man with high triglycerides serum levels for 3-years refractory to treatment with fibrates (Gemfibrozil 600mg/12h). He was asymptomatic, a sportsman, with normal BMI and blood pressure, no previous history of cardiovascular disease, and he is not diabetic. The patient reported that he did not smoke, drink alcohol or undertake fat diet. The patient's medical history showed a triglyceride concentration for 638mg/dl and 497mg/dl 12 months ago. Familial history of cardiovascular disease was reported. Parents showed normal serum triglyceride levels and no previous cardiovascular events.
MethodsSerum and plasma (EDTA) were collected after a 12-h overnight fasting for routine laboratory tests. Biochemistry parameters, cholesterol and triglycerides serum levels were assayed on automated Advia 2400 Analyzer (Siemens Healthcare Diagnostics, Germany). TSH was measured on Advia Centaur XP Analyzer (Siemens Healthcare Diagnostics, Germany). Cardiovascular risk profile including the emerging risk factors (fibrinogen and Lp(a)) and apolipoproteins (ApoA-I and ApoB-100), was measured on BN ProSpec Analyzer (Siemens Healthcare Diagnostics, Germany) and on Roche Cobas e601 Analyzer (Roche Diagnostics, Mannheim, Germany). VLDL particles (d<1.006g/ml) were isolated after ultracentrifugation, and the HDL subfraction was obtained after precipitation of LDL (d>1.006g/ml) with dextran sulfate and magnesium chloride. Serum glycerol was measured with an enzymatic method (Boehringer Mannheim/R-biopharm, Germany). Written consent was obtained from the patient to publish this report.
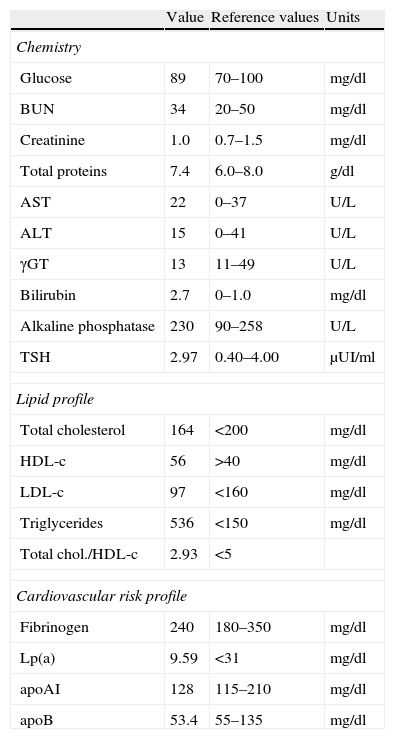
ResultsInitial laboratory investigations showed high levels of triglycerides. These levels were maintained over a three-year follow-up. Serum glucose, creatinine, alanine aminotransferase, aspartate aminotransferase, and thyroid hormone values were within reference limits. Cardiovascular risk profile was normal (Table 1). Analytical parameters such as lipaemia and hemolysis were within acceptable limits. Serum lipoprotein was separated in density gradient and electrophoresis revealed qualitative and quantitative normal lipoproteins (very low density lipoprotein (VLDL) particles triglycerides-rich and partially delipidated, normal and low density LDL and normal HDL). Serum glycerol determination revealed 4.17mmol/l (reference interval, 0.03–0.19mmol/l), equivalent to about 360mg/dl of serum triglycerides.
Serum levels of biochemical parameters.
| Value | Reference values | Units | |
| Chemistry | |||
| Glucose | 89 | 70–100 | mg/dl |
| BUN | 34 | 20–50 | mg/dl |
| Creatinine | 1.0 | 0.7–1.5 | mg/dl |
| Total proteins | 7.4 | 6.0–8.0 | g/dl |
| AST | 22 | 0–37 | U/L |
| ALT | 15 | 0–41 | U/L |
| γGT | 13 | 11–49 | U/L |
| Bilirubin | 2.7 | 0–1.0 | mg/dl |
| Alkaline phosphatase | 230 | 90–258 | U/L |
| TSH | 2.97 | 0.40–4.00 | μUI/ml |
| Lipid profile | |||
| Total cholesterol | 164 | <200 | mg/dl |
| HDL-c | 56 | >40 | mg/dl |
| LDL-c | 97 | <160 | mg/dl |
| Triglycerides | 536 | <150 | mg/dl |
| Total chol./HDL-c | 2.93 | <5 | |
| Cardiovascular risk profile | |||
| Fibrinogen | 240 | 180–350 | mg/dl |
| Lp(a) | 9.59 | <31 | mg/dl |
| apoAI | 128 | 115–210 | mg/dl |
| apoB | 53.4 | 55–135 | mg/dl |
This is a clinical case of a patient with a possible deficiency of glycerol kinase with elevated serum glycerol but asymptomatic. He was refractory to fibrate treatment during follow-up. Interestingly, serum appearance, lipemic markers and lipid profile observed with the serum lipoprotein separation were normal. This made us think in an interfering component with triglycerides assay. Other authors (Fodor et al.3) also had reported an interference of glycerol with plasma ethylene glycol determination.
Glycerol is a halfway product in triglycerides determination. Most enzymatic methods used in routine laboratories do not involve a glycerol blank and only determine triglycerides levels because free glycerol concentrations in serum fasting individuals are small, and are considered normal and in the range of 0.01–0.10mmol/l,4 so do not interfere with the quantification of total triglycerides; for this, most methods do not use a “white glycerol.” In order to eliminate the risk of false classifications of hypertriglyceridaemia, there are methods used in autoanalyzers which remove free glycerol by an enzymatic reaction prior to the addition of lipase for the hydrolysis of triglycerides. Thereby once separated, we will measure only glycerol in a reaction catalyzed by glycerokinase (GK).5 In this analysis, the amount of NADH oxidized in the above reaction is stoichiometric to the amount of glycerol. These methods could eliminate the risk of false classifications of hypertriglyceridaemia.
The high glycerol levels obtained made us suspect in hyperglycerolemia but genetic test of GK gene should be included to determine the definitive diagnosis.6
The glycerol kinase deficiency is a metabolic disorder characterized by glycerol between 1.8 and 8.0mmol/l and between and gliceroluria 360mmol/24h. It is a very rare X-linked recessive genetic disorder caused by mutations in the glycerol kinase gene (GK) (OMIM 307030). The product of this gene catalyzes the phosphorylation of glycerol by ATP, yielding ADP and glycerol-3-phosphate. Consequently, glycerol kinase deficiency (GKD) produces high serum and urine glycerol levels noted that there are 3 clinically distinct forms of GKD7: infantile, juvenile, and adult. The infantile form is associated with severe developmental delay and results in a syndrome with Xp21 gene deletion with congenital adrenal hypoplasia and/or Duchenne muscular dystrophy. GKD occurs in two forms:
- (1)
complex: GKD involving a deletion of the GK gene, together with the gene for Duchenne muscular dystrophy and/or congenital adrenal hypoplasia, and is associated with severe symptoms, including cortisol deficiency and salt-wasting symptoms.8 In one patient with deletion in Xp21, further laboratory findings defined high glycerol concentrations both in blood and in urine that were compatible with a glycerol kinase deficiency (GKD), hypoadrenalism (hyponatremia, hyperpotasemia, dehydration), high creatine phosphokinase level. The isolated form is usually entirely asymptomatic9–11 and often is detected in grown-up individuals because of therapy-resistant pseudohypertriglyceridaemia. It would be the type of our patient. Nevertheless, the juvenile and adult forms are often detected fortuitously due to having no symptoms and carry an isolated GKD.12
Wibmer et al.13 informed a case with pseudohypertriglyceridaemia refractory to lipid-lowering therapy for more than 15 years revealing an isolated and asymptomatic glycerol kinase deficiency as an another children in our laboratory,4 children of 6 years old with hypertriglyceridemia than 300mg/dl, with a weight of 22kg, a size and, body mass index normal and no history of other diseases. Glycerol analysis showed 3.81mmol/l, values lower than our patient.
For all these reasons, we suggested the withdrawal of treatment, due to fibrates having no effect on free glycerol.6 Furthermore, we have to take into account that glycerol is a part of gluconeogenesis, a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates. Children with GKD may have severe hypoglycemic episodes and profound metabolic acidosis, or be totally free of symptoms.11,14 These individuals are unable to form glucose from the glycerol released during triglyceride catabolism aldo the hypoglycemic episodes often disappear during adolescence, which is linked to increased hepatic gluconeogenic capacity.4
Therefore, patients should be careful to prolonged fasting or strenuous sports that could trigger a ketonic hypoglycemia. After puberty, if the individual has been asymptomatic, a fasting test of physical capacity would give objective information on the degree of metabolic compensation.15
In conclusion, it may be important to consider glycerol kinase deficiency in patients with elevated triglyceride concentrations and asymptomatic for differential diagnosis of hypertriglyceridaemia resistant to treatment with fibrates. Although clinical symptoms in adults are extremely rare, asymptomatic patients should be advised of possible extreme catabolic situations.
Potential conflict of interestThe authors declare no conflicts of interest.
Clinical case awarded at the Second Congress of hypertriglyceridemia. La Granja de San Francisco, Segovia, Spain.