Abdominal aortic aneurysm (AAA) shares several risk factors with atherosclerosis. Among these, diabetes mellitus (DM) could have a negative effect on the formation, growth and expansion of AAA. Several systematic reviews and meta-analyses reported up to 2016 have shown concordant results regarding the possible protective effect on AAA formation.
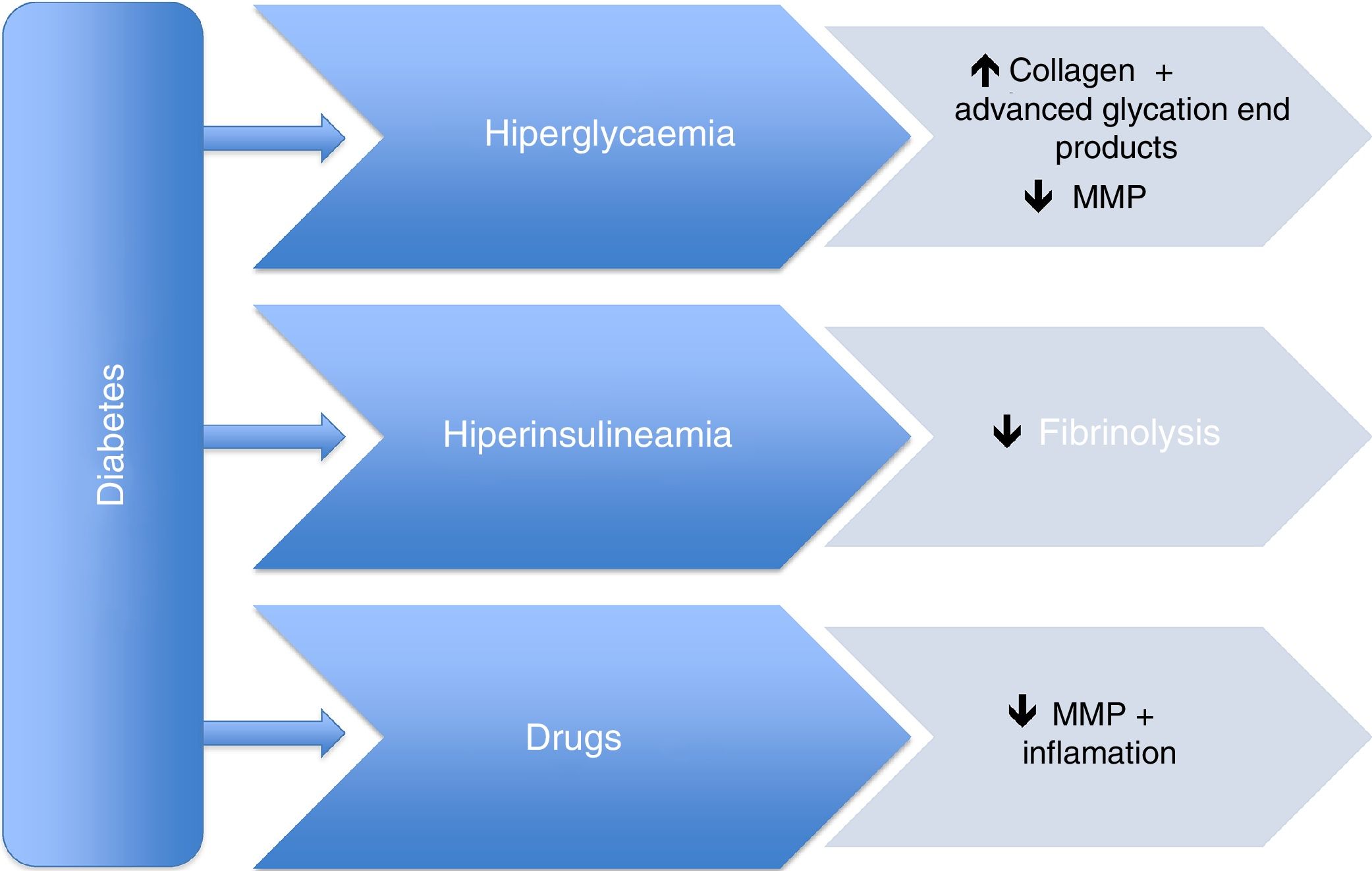
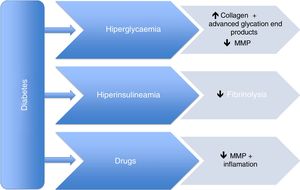
However, the pathophysiological mechanisms of this supposed protective effect are still unknown. It appears that both hyperglycaemia and hyperinsulinemia, which are closely associated with DM, cause an increase in advanced glycation end-products, a decrease in fibrinolysis, and alterations in smooth muscle cells, leading to a decreased risk of aneurysm growth and expansion. This protective role is mainly mediated by a decrease in metalloproteinases, mainly type 2 and 9. In addition, drugs used to treat type 2 DM, as well as those for hypertension and dyslipidaemia could also play an important role in this protective effect. Metformin, thiazolidinediones, DPP4 inhibitors, statins, and angiotensin-converting enzyme inhibitors have been evaluated in this field.
In conclusion, studies reported so far seem to confirm the protective effect of DM in both the formation and expansion of AAA, although future long-term studies are needed to confirm the pathophysiological mechanisms involved, as well as the role of concomitant medication.
El aneurisma de aorta abdominal (AAA) comparte diversos factores de riesgo con la aterosclerosis. De entre estos la diabetes mellitus (DM) podría tener un efecto negativo en la formación, crecimiento y expansión del AAA. En este sentido, diversas revisiones sistemáticas y metaanálisis publicados hasta 2016 han mostrado resultados concordantes en cuanto al posible efecto protector de la DM en la formación y progresión del AAA.
No obstante, los mecanismos fisiopatológicos de esta supuesta protección son aún desconocidos. Parece que tanto la hiperglucemia como la hiperinsulinemia asociadas a la DM, al causar un incremento de los productos de glicación avanzada, una disminución de la fibrinólisis y alteraciones en las células musculares lisas, comportarían un menor riesgo de expansión aneurismática. Se considera que este papel protector está principalmente mediado por una disminución de las metaloproteasas, en concreto la tipo 2 y 9. Además, los fármacos utilizados en el tratamiento tanto de la DM tipo 2 como de la hipertensión arterial y la dislipidemia también podrían desempeñar un papel en este efecto protector. La metformina, las tiazolidinedionas, los inhibidores del DPP4, las estatinas y los inhibidores de la enzima conversora de la angiotensina han sido evaluados en este sentido.
En conclusión, los estudios publicados hasta el momento parecen confirmar el efecto protector de la DM en la formación y expansión del AAA, aunque son necesarios futuros estudios a largo plazo para confirmar los mecanismos fisiopatológicos implicados, así como el papel de la medicación concomitante.
An abdominal aortic aneurysm (AAA) is a disease with a significant family burden which shares several cardiovascular risk factors with atherosclerosis, such as: old age (>60), male gender, white race, hypertension, hypercholesterolaemia, at the expense of low-density lipoproteins (LDL), smoking, advanced atherosclerotic disease, obesity, as well as a family or personal history of cardiovascular disease.1 However, although diabetes mellitus (DM) is a risk factor for the development of coronary heart disease, cerebrovascular disease and peripheral arterial disease, it could have a negative effect on the formation, growth and expansion of an AAA. In this regard, previous studies have suggested a possible protective effect of DM in the prevalence of AAA.2,3 These results are in line with those reported in two previous meta-analyses.4,5 However, the pathogenic link between an AAA and DM is unknown, and the possible pathophysiological mechanisms of this supposed protective effect have not yet been established.
The normal diameter of the aortic artery varies depending on age, gender and body weight, and its calibre decreases gradually until the iliac arteries bifurcate.6 An AAA is defined as a pathological dilation of the aorta greater than 3cm, with the infrarenal location being the most common. The increased morbidity and mortality in the adult population associated with aortic aneurysms is well-known, especially when they are located in the abdomen. The natural history of the disease tends to be silent until some of its complications develop: rupture, with a probability directly proportional to the size and speed of growth of the aneurysm, formation of a thrombus in the aortic wall or the compression of adjacent organs.7 However, mortality from an AAA and aortic dissection have remained stable in recent years, probably due, at least partly, to the notable progress in diagnostic-therapeutic tools. In Spain, a total of 1876 patients died in 2013 due to the presence of an AAA or aortic dissection (ICD-10 I71),8 1477 of whom were male.
Traditionally, AAA has been considered a focal manifestation of advanced atherosclerotic disease.9 However, new evidence indicates that an AAA is a localised form of a systemic vascular disease, characterised by a progressive loss of the aortic wall's resistance capacity due to increased intraluminal pressure and a progressive degradation of the aortic wall.
We have therefore deemed it relevant to analyse the existing evidence on the association between DM and AAA, and to describe the possible pathophysiological mechanisms involved in the supposed protective effect. The possible association between the medication used for type 2 DM, hypertension or dyslipidaemia, and its effect on the progression of aneurysms, has also been reviewed.
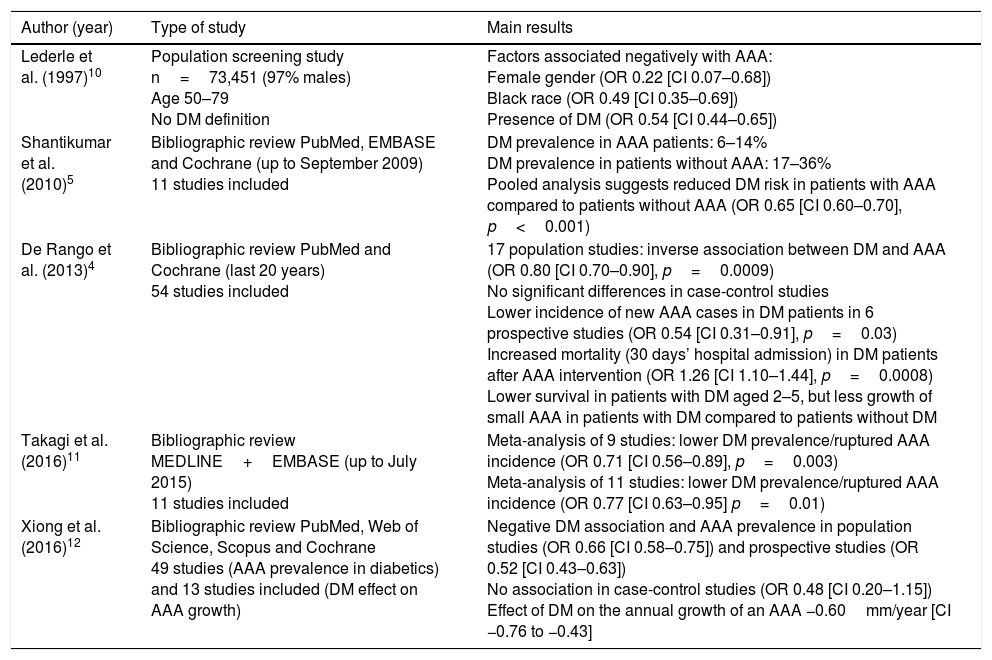
Clinical evidenceOne of the first studies which underlined the possible negative association between DM and AAA was that carried out by Lederle et al.10 in 1997. Based on the Aneurysm Detection and Management (ADAM) study, they analysed 73,451 patients with no previous history of AAA, aged between 50 and 79, 97% of whom were male. They demonstrated that the female gender (odds ratio [OR] 0.22 [confidence interval (CI) 0.07–0.68]), black race (OR 0.49 [CI 0.35–0.69]) and the presence of DM (OR 0.54 [CI 0.44–0.65]) were associated negatively with AAA.
After this seminal study, several systematic reviews and meta-analyses have reproduced similar findings. In this regard, Shantikumar et al.5 included 11 studies, up to September 2009, with the main objective of evaluating the association between AAA and DM, and establishing the pathophysiological mechanisms associated with the supposed protective effect. The prevalence of DM in patients with an AAA was 6%–14%, and the prevalence of DM in patients in the control group without an AAA was 17%–36%. Furthermore, the results suggested a reduction in the rate of DM in patients with an AAA in comparison to those without aortic disease (OR 0.65 [CI 0.60–0.70], p<0.001). Along the same lines, the meta-analysis by De Rango et al.4 included 54 studies conducted in the last 20 years, independently assessing the population screening, case-control and prospective studies to maintain homogeneity among them. In the 17 prevalence studies, an inverse association was observed between DM and AAA (OR 0.80 [CI 0.70–0.90] p=0.0009), while no significant differences were observed in the case-control studies. Similarly, a reduced incidence of de novo AAA was observed in patients with DM in six prospective studies (OR 0.54 [CI 0.31–0.91], p=0.03). Finally, patients with DM had increased mortality 30 days after hospital admission for AAA surgery (OR 1.26 [CI 1.10–44], p=0.0008).
It is important to highlight the findings of two systematic reviews carried out in the last two years. First, Takagi et al.11 published a systematic review of the studies published up to July 2015, including a total of 11 studies which evaluated the association between AAA and DM. A first meta-analysis included nine studies with patients with a ruptured AAA, demonstrating that DM was associated with a lower prevalence/incidence of ruptured AAA (OR 0.71 [CI 0.56–0.89], p=0.003). A second meta-analysis of 11 studies concluded that DM was associated with a lower prevalence/incidence of ruptured AAA (OR 0.77 [CI 0.63–0.95], p=0.01). Lastly, the meta-analysis by Xiong et al.12 included 49 studies with the main objective of assessing the effect of DM on the presence and growth of an AAA. A negative association was therefore observed between DM and the prevalence of AAA in population screening studies (OR 0.66 [CI 0.58–0.75]) and prospective studies (OR 0.52 [CI 0.43–0.63]) but, once again, not in the case-control studies (OR 0.48 [CI 0.20–1.15]). The effect of DM on the annual growth of an AAA was −0.60mm/year (CI −0.76 to −0.43).
In summary, the different systematic reviews and meta-analyses published up to 2016 (Table 1) show concordant results regarding the possible protective effect of DM on AAA formation and progression.
Main studies evaluating the effect of DM on AAA.
| Author (year) | Type of study | Main results |
|---|---|---|
| Lederle et al. (1997)10 | Population screening study n=73,451 (97% males) Age 50–79 No DM definition | Factors associated negatively with AAA: Female gender (OR 0.22 [CI 0.07–0.68]) Black race (OR 0.49 [CI 0.35–0.69]) Presence of DM (OR 0.54 [CI 0.44–0.65]) |
| Shantikumar et al. (2010)5 | Bibliographic review PubMed, EMBASE and Cochrane (up to September 2009) 11 studies included | DM prevalence in AAA patients: 6–14% DM prevalence in patients without AAA: 17–36% Pooled analysis suggests reduced DM risk in patients with AAA compared to patients without AAA (OR 0.65 [CI 0.60–0.70], p<0.001) |
| De Rango et al. (2013)4 | Bibliographic review PubMed and Cochrane (last 20 years) 54 studies included | 17 population studies: inverse association between DM and AAA (OR 0.80 [CI 0.70–0.90], p=0.0009) No significant differences in case-control studies Lower incidence of new AAA cases in DM patients in 6 prospective studies (OR 0.54 [CI 0.31–0.91], p=0.03) Increased mortality (30 days’ hospital admission) in DM patients after AAA intervention (OR 1.26 [CI 1.10–1.44], p=0.0008) Lower survival in patients with DM aged 2–5, but less growth of small AAA in patients with DM compared to patients without DM |
| Takagi et al. (2016)11 | Bibliographic review MEDLINE+EMBASE (up to July 2015) 11 studies included | Meta-analysis of 9 studies: lower DM prevalence/ruptured AAA incidence (OR 0.71 [CI 0.56–0.89], p=0.003) Meta-analysis of 11 studies: lower DM prevalence/ruptured AAA incidence (OR 0.77 [CI 0.63–0.95] p=0.01) |
| Xiong et al. (2016)12 | Bibliographic review PubMed, Web of Science, Scopus and Cochrane 49 studies (AAA prevalence in diabetics) and 13 studies included (DM effect on AAA growth) | Negative DM association and AAA prevalence in population studies (OR 0.66 [CI 0.58–0.75]) and prospective studies (OR 0.52 [CI 0.43–0.63]) No association in case-control studies (OR 0.48 [CI 0.20–1.15]) Effect of DM on the annual growth of an AAA −0.60mm/year [CI −0.76 to −0.43] |
AAA: abdominal aortic aneurysm; CI: 95% confidence interval; DM: diabetes mellitus; OR: odds ratio.
The pathophysiological mechanisms responsible for the negative association between DM and AAA are still not well defined. Therefore, we present below the most relevant information from the in vitro and in vivo studies.5,13
The aortic wall of aneurysms has reduced levels of collagen and elastin due to increased proteolytic activity. This alteration is closely linked to an increase in proteolytic enzymes, specifically to matrix metalloproteinases (MMPs). Within this group of proteases, MMP type 2 (MMP-2) of the smooth muscle cells (SMC) and type 9 (MMP-9),14,15 which come from macrophages, are responsible for matrix protein and arterial wall degradation. In fact, elevated levels of MMP-2 and MMP-9 have been reported in patients with an AAA, and the lack of growth of aneurysms in the aorta has also been confirmed in animal models deficient in these two MMPs.16
DM promotes an increase in the synthesis and a reduction in the degradation of the extracellular matrix, with the subsequent lower risk of aneurysms forming.13 Moreover, in vitro studies have revealed that hyperglycaemia increases collagen synthesis,17 with the resulting increase in the thickness of the aortic wall and a concomitant reduction in MMP levels. In fact, the thickness of the aortic wall in patients with DM is greater than in patients without DM, and a study in humans observed a reduction in MMP-2 and MMP-9 in the coronary arteries of patients with DM.18 Therefore, a similar decrease in these MMP sub-classes in the aneurysm aortic wall of patients with DM could explain the negative effect on AAA growth and development.
Furthermore, patients with DM tend to present with hyperglycaemia and hyperinsulinaemia. This leads to an increase in advanced glycation end products in the extracellular matrix, with a binding of these to the elastin and collagen of the arterial wall, also promoting SMC proliferation.19,20 All these changes contribute to the aortic wall being firmer and more resistant and, therefore, to a reduced risk of aneurysm formation. However, other studies have shown contradictory results, suggesting that the presence of advanced glycation end products could be associated with an increase in MMPs and other inflammatory cytokines and, therefore, promoting AAA formation.21,22
In addition, the SMC alterations could explain, at least partly, the pathogenesis of AAA. In a recent study, in vitro morphological differences were noted between the SMCs of patients with type 2 DM compared to patients with a strictly normal hydrocarbon metabolism.23 This is due to an increase in vinculin, the protein which binds the extracellular matrix to the cytoskeleton. This increase in vinculin could be responsible for the marked rigidity of the aortic wall in patients with DM,24 thereby conferring a lower risk of aneurysm formation.
The alteration of the fibrinolytic system within the aortic wall could also play an essential role. A previous study observed that the fibrous cap was altered in patients with DM. It was denser, less porous and more resistant to fibrinolysis.25 It is possible that hyperinsulinism leads to an increase in fibrinogen and the plasminogen activator inhibitor type 1 (PAI-1). The latter acts mainly by inhibiting the conversion of plasminogen to plasmin, and therefore suppressing the fibrinolytic system. Furthermore, plasmin is responsible for the conversion of pro-matrix metalloproteinases to their active form, related to AAA formation.26,27 Therefore, increased levels of PAI-1 as a result of hyperinsulinaemia in patients with DM could facilitate the stabilisation of the aortic wall, preventing the formation of aneurysms by reducing MMP levels.28
Therapeutic agentsIt has been speculated that the supposed protective effect of DM in the formation of AAAs could be due not only to the alterations of the above-mentioned pathophysiological mechanisms, but also to the drugs used for the control of several cardiovascular risk factors present in patients with DM. It seems that there could be a link not only to the treatment used for type 2 DM, but also to the treatment used for hypertension and dyslipidaemia. Many of these patients, with an increased cardiovascular risk, share these three risk factors and use active medication for these diseases.
Previous studies have already focussed on this point. In 2010, Thompson et al.29 demonstrated that some drugs used to treat type 2 DM reduced the size of the AAA regardless of insulin. Other studies have shown similar results. We will now present the evidence that exists on several drugs regarding their effect on the development of aortic aneurysms. Fig. 1 displays the main pathophysiological mechanisms, as well as the action of the concomitant medication.
Medication used in type 2 diabetes mellitusFirst, a possible protective role of metformin in AAA development has been described. This drug, considered to be a first-line drug for the start of drug treatment in patients with type 2 DM, leads to a reduced vascular risk in these patients, but it also seems to play a complementary role in the aorta. In vitro studies have reported that metformin inhibits the effect of leptin on SMC proliferation and MMP-2 levels, with a consequent reduction in MMP levels and a lower SMC proliferation of the aortic wall in humans, and thereby preventing the formation of aneurysms.30
Another therapeutic drug group for type 2 DM, thiazolidinediones, could play a role in aneurysm development. In this regard, rosiglitazone has demonstrated in animal models that it reduces MMP-9 levels, as well as other pro-inflammatory markers, such as C-reactive protein.31,32 These changes could reduce the risk of AAA and play an important overall role in reducing the cardiovascular risk of these patients.
Another pharmacological group which has been introduced more recently in DM treatment, DPP-4 inhibitors, could also present a negative effect regarding aneurysm development. Studies in animal models have observed a reduction in AAA formation and expansion when receiving treatment with alogliptin. This effect is dose-dependent and attributed to an antioxidant effect.33 In another more recent study with animal models, sitagliptin showed a beneficial effect in the formation of aortic aneurysms, by increasing GLP-1 activity, with a reduction in MMP-2 and MMP-9.34
Therefore, it seems that the beneficial effect of DM in terms of the formation and progression of aortic aneurysms could be mediated by the drug treatment used in these patients, and not only by the presence of type 2 DM on its own.
Lipid-lowering drugsWith regard to the drug treatment for dyslipidaemia, statins have been linked to a negative effect in the formation of aneurysms. Several trials with these lipid-lowering drugs have been carried out, both in animal models and in humans, observing reduced levels of MMP in the aortic wall,35 as well as a reduction in AAA expansion, regardless of other risk factors related to aneurysm growth.36
Antihypertensive drugsAngiotensin-converting enzyme inhibitors, drugs which are widely used as antihypertensive treatment, have demonstrated that they could play a protective role in AAA formation. The main effect involved lies in a reduction in vascular inflammation, an increase in elastin deposits and an inhibition of MMPs,37 although all the related pathophysiological mechanisms are still to be confirmed. In animal models, this therapeutic group has managed to inhibit AAA expansion and rupture, regardless of the haemodynamic effect of these drugs to reduce blood pressure.38
ConclusionsA large part of the clinical evidence described in this review confirms the protective effect of DM in AAA formation and expansion. However, these studies do not always differentiate the different types of DM, a factor which could play a significant role in the apparent protection. Furthermore, experimental studies indicate that the pathophysiological mechanisms involved are closely related to MMP-2 and MMP-9. Nevertheless, concomitant medication to treat type 2 DM, hypertension and dyslipidaemia could also play a role. More studies evaluating the impact of DM, and especially of the concomitant medication in the evolution of AAA, are therefore needed to obtain more solid conclusions in this field.
Conflicts of interestNone.
Please cite this article as: Climent E, Benaiges D, Chillarón JJ, Flores-Le Roux JA, Pedro-Botet J. La diabetes mellitus como factor protector del aneurisma de aorta abdominal: posibles mecanismos. Clin Invest Arterioscler. 2018;30:181–187.