The corpus luteum (CL) is a transitory endocrine structure found in female mammals which secretes progesterone (P4), rendering the endometrium secretory and thus facilitating embryo implantation in the uterus. CL insufficiency (CLI) is defined as a condition in which CL P4 cannot maintain the endometrium and gestation, causing infertility and pregnancy loss. The present study aimed to compare specific aspects related to CLI in humans and domestic animals to identify its main aetiological differences and those related to treatment of the condition in different species.
El cuerpo lúteo (CL) es una estructura endocrina transitoria que se encuentra en las hembras de mamíferos y que secreta progesterona (P4), volviendo al endometrio secretor y facilitando así la implantación del embrión en el útero. La insuficiencia de CL se define como una condición en la que CL P4 no es capaz de mantener el endometrio y la gestación, provocando infertilidad y pérdida del embarazo. El presente estudio tuvo como objetivo comparar aspectos específicos relacionados con la insuficiencia del CL en humanos y animales domésticos con el fin de identificar sus principales diferencias etiológicas y las relacionadas con el tratamiento de dicha condición en distintas especies.
In both humans and domestic animals, the CL is a transient endocrine gland1,2 formed as a result of ovulation and the action of luteinizing hormone (LH) on follicular theca interna and granulosa cells3 and other luteotrophic hormones, such as prolactin and estradiol in rodents.1
The CL is associated with hormone secretion, including P4, which acts on the endometrium, making it secretory and thus facilitating embryo implantation,1,2,4 as well as maintaining the endometrial lining at the beginning of pregnancy until placenta takes over this role.1,3,5
The duration of the luteal phase is defined as the interval after the peak of the LH, until the day before the start of menstruation.6 This phase in non-human primates extends from 12 to 16 days, depending on the species,7 whereas in humans this phase lasts from 13 to 15 days.8
Luteal phase deficiency is characterized as a condition where the CL is unable to secrete sufficient amounts of P4 to maintain a secretory endometrium and allow normal embryo implantation and growth.6
Thus, the present study aimed to carry out a comparative survey of specific aspects related to the insufficiency of CL in humans and domestic animals to identify the etiological differences and those related to the treatment of this condition.
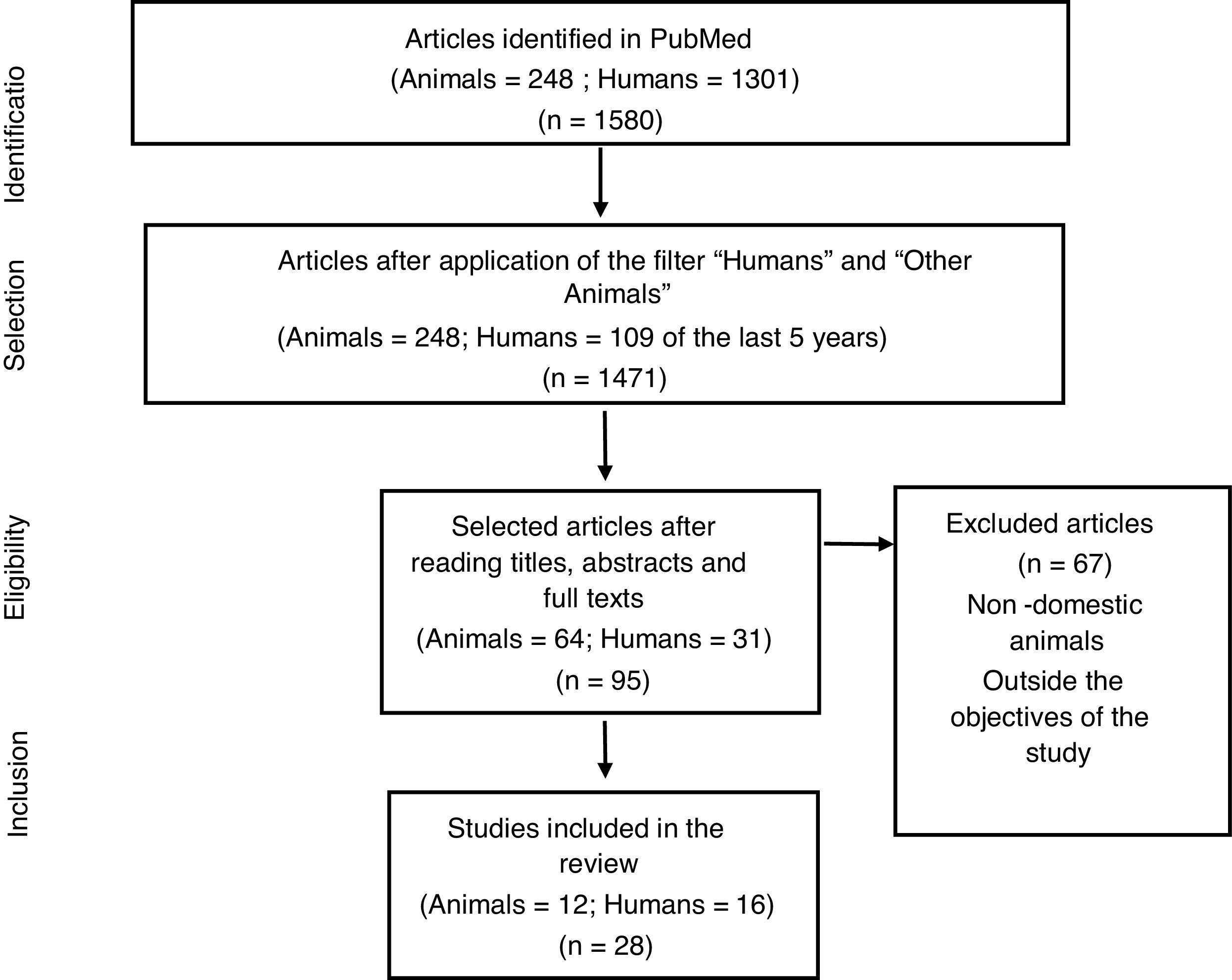
MethodsThis is a comparative study on CLI between humans and animal species, carried out by searching for articles in PubMed, in October 2020, with the descriptors Luteal insufficiency, Luteal phase insufficiency, Luteal phase deficiency or Luteal phase defect in English. The filters “Humans” and “Other Animals” were used to select articles for each of the species addressed in the study, totaling 248 studies with animals and 1301 with humans. For animal studies, few articles were found in the database. Thus, it was decided to analyze all available studies. In human studies, due to the large number of articles available, it was decided to establish a time frame of the last 5 years, totaling 109 articles. Soon after reading the titles and respective abstracts, articles that were not suitable for this study were excluded and 28 articles being used (Fig. 1). The selection of articles was carried out by one of the authors (Leonardo Garcia Góes) and checked by the others.
Results and discussionIn humans and domestic animals, CL is formed after ovulation under the action of luteotrophic hormones1,3 and is associated with P4 secretion that has a fundamental role in the beginning of pregnancy.1,2,4
CLI is defined as a condition where the P4 produced by the CL is not sufficient to maintain the endometrium and pregnancy,3 thus being related to infertility and early pregnancy loss in humans.3–5,8 In dogs, in addition to these manifestations,9 shorter estrous cycles may be present.10 In these animals, the abnormal decrease in P4 concentrations can occur at the end of estrus (post-ovulation) or at any time during diestrus (pregnant or not).11
In humans, premature onset of menstruation is an indicative of luteal phase deficiency4,12 and a lag of more than 2 days in the histological development of the endometrium compared to the expected day of the cycle is the most accepted consensus on a defective luteal phase.4,13
In dogs, this deficiency is defined as a decrease in serum P4 concentrations to <2ng/mL before the normal end of the diestrus (day 65).11 In relation to pregnancy, a female dog can be considered with luteal phase deficiency when the serum P4 is below 5ng/mL in the 4–5th week of pregnancy, in the absence of infectious or metabolic disease.14
In humans, CLI may be related to conditions such as thyroid dysfunction, hyperprolactinemia, insufficient secretion of LH, primary dysfunction of the CL2,6 external factors such as air pollution,15 dietary factors16 or related to ovulation induction therapies for in vitro fertilization cycles.2,17–19 Low FSH levels, as well as high levels of estradiol in the initial follicular phase are also related to luteal phase deficiency. In addition, the risk of a short luteal phase decreased with the increase in inhibin B.12
In a study that evaluated the association between ovarian reserve biomarkers (anti-Müllerian hormone, FSH, inhibin B and estradiol) and luteal phase deficiency in humans, Pfister, Crawford and Steiner12 concluded that there was no association between low ovarian reserve and luteal phase of short duration.
In dogs, this insufficiency seems to be related to primary alterations of the CL.10 In non-human primates it is related to an inadequate pattern of FSH secretion7,20 and LH,20 in addition to frequent breastfeeding before ovulation and during the subsequent luteal phase.21 In cows22 and sheep,23 as well as in humans, it is also associated with ovulation induction therapies.
Other factors may be associated with CL failure. A study by Krachudel et al.10 showed that, in German sheepdogs, prolactin concentrations were significantly higher in pregnant bitches with luteal hypofunction compared to the control group. Besides, increased levels of IgE antibodies against P4 have been found in the serum of some short-cycle bitches, suggesting that these antibodies may play a role in some cases of unexplained pregnancy failure and shortened luteal phase.
In the dog, the CL is the only source of circulating P4 during pregnancy. Thus, inadequate luteal function can be both a cause of recurrent miscarriage or early fetal resorption,14 as well as premature delivery and abortion during the second half of pregnancy.10,14 In humans, on the other hand, this condition is related to abortions, defined as pregnancy losses in the first half of pregnancy.3–5
Changes resulting from CLI, in addition to causing repercussions in the current cycle, can also influence gonadal function during subsequent reproductive cycles, as presented in a study by Wilks, Hodgen and Ross,20 which evaluated the hormonal profile of Rhesus monkeys with the occurrence of excessively long menstrual cycles after the cycle with a short luteal phase, probably related to the fact that the formation of a functional CL results from an adequate folliculogenesis.
Regarding the treatment of this condition in humans, the correct approach is the identification and correction of possible correlated conditions and, if not, empirical treatment must be performed.17 Progestogens that mimic P4 activity have been used in an attempt to overcome this hormone deficiency associated with infertility and spontaneous abortion.4,5
A study carried out by Orazov et al.,6 which evaluated 35 women in reproductive age diagnosed with CLI, showed that the combined therapy of P4 and metabolic correction with placental hydrolyzate contributed to the recovery of cyclic events in the gonadal axis, restoring ovarian function.
In humans, despite isolated studies showing efficacy in the use of P4, there is still insufficient data to prove the efficacy of the supportive treatment of the luteal phase in spontaneous ovulatory cycles, not induced by gonadotropins.13,17
On the other hand, in cases related to ovulation induction for in vitro fertilization cycles, P4 is indicated, having a positive effect on reproductive results.13,17,18 The exogenous administration of P4 in the form of vaginal tablets, creams or suppositories is currently the most recommended in humans.19,24
Another treatment proposed for humans is the daily application of hCG in low doses.19,25 However, this alternative is not yet widespread, since recommended dosages are not commercially available.19 However, information regarding P4 administration protocols, routes of administration, dose and possible associations with other drugs is not yet a consensus.
A multicenter, randomized, double-blind, placebo-controlled study conducted at 36 hospital centers in the United Kingdom and 9 in the Netherlands evaluated women who had recurrent and unexplained abortions and had received micronized 400mg vaginal P4 or vaginal placebo capsules twice a day, soon after positive urine pregnancy test up to 12 full weeks of gestation. There was no evidence that therapy improved outcomes in these patients.26
In domestic animals, P4 supplementation also appears to be effective for the management of suspected luteal deficiency. The recommendation is to start supplementation if P4 concentrations are below 5ng/mL before the last week of pregnancy and to avoid the early administration of progestogens, due to the risk of teratogenicity if administered at the beginning of pregnancy.9
As in humans, treatment with hCG during the early luteal phase contributed significantly to maintaining pregnancy and increasing the calving rate in Simmental cows.27
Despite data from recents articles in humans presented in this study, the Practice Committee of the American Society for Reproductive Medicine shows that the “luteal phase deficiency has been described in healthy normally menstruating women and, although progesterone is important for the process of implantation and early embryonic development, luteal phase deficiency, as an independent entity causing infertility, has not been proven”.28
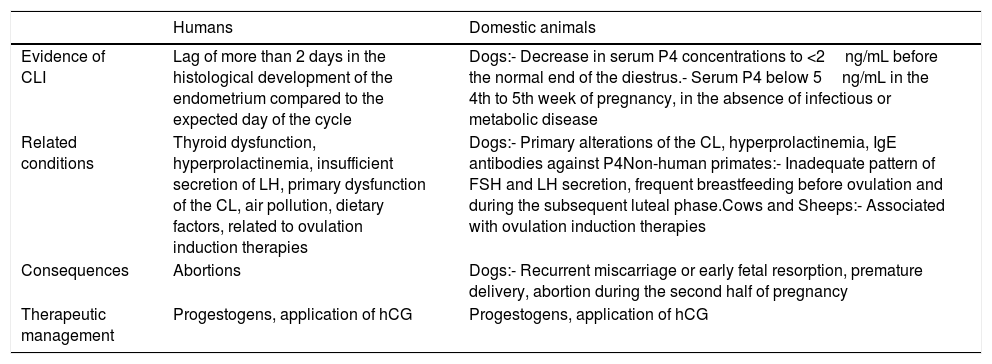
In summary, the main information regarding the insufficiency of luteum corpus in humans and domestic animals is presented in Table 1.
Comparative table between humans and domestic animals.
| Humans | Domestic animals | |
|---|---|---|
| Evidence of CLI | Lag of more than 2 days in the histological development of the endometrium compared to the expected day of the cycle | Dogs:- Decrease in serum P4 concentrations to <2ng/mL before the normal end of the diestrus.- Serum P4 below 5ng/mL in the 4th to 5th week of pregnancy, in the absence of infectious or metabolic disease |
| Related conditions | Thyroid dysfunction, hyperprolactinemia, insufficient secretion of LH, primary dysfunction of the CL, air pollution, dietary factors, related to ovulation induction therapies | Dogs:- Primary alterations of the CL, hyperprolactinemia, IgE antibodies against P4Non-human primates:- Inadequate pattern of FSH and LH secretion, frequent breastfeeding before ovulation and during the subsequent luteal phase.Cows and Sheeps:- Associated with ovulation induction therapies |
| Consequences | Abortions | Dogs:- Recurrent miscarriage or early fetal resorption, premature delivery, abortion during the second half of pregnancy |
| Therapeutic management | Progestogens, application of hCG | Progestogens, application of hCG |
A limitation identified in this study is the fact that the bibliographic search for articles in humans covers a short period of time. Thus, a broader literature review is suggested to corroborate or not with the findings of this study.
ConclusionsThe data presented show a great similarity in relation to the shortcomings of the CL in humans and domestic animals, being related to recurrent abortions and infertility, as a consequence of primary alterations of the CL, inadequate secretion of gonadotrophic hormones and to ovulation induction therapies.
Regarding the therapeutic management of this condition, in veterinary medicine, studies, which are still scarce, suggest the administration of progestogens to support the luteal phase. In humans, on the other hand, the use of luteal phase support with progestogens is already well defined only in the insufficiency of the CL related to ovulation induction.
Through this comparative study, researchers in human and veterinary medicine can outline new studies, to elucidate and manage conditions present in their field of action through common and divergent knowledge between the species presented.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.