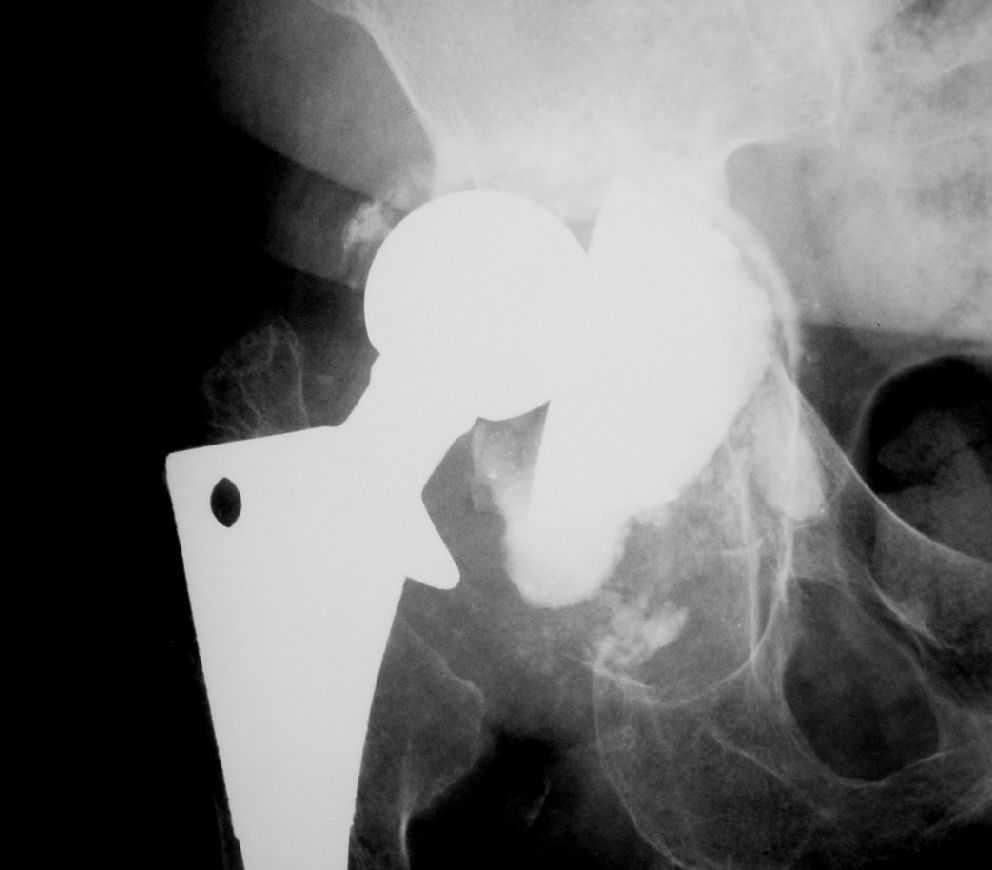
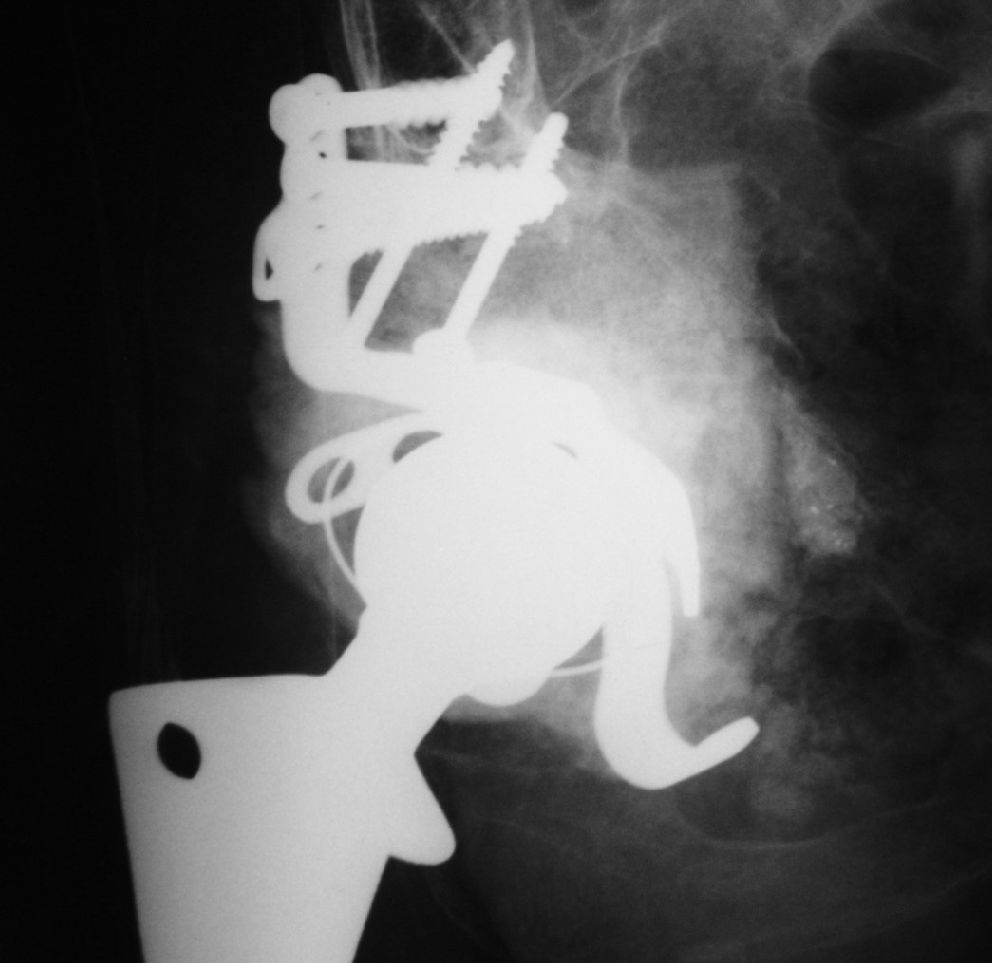
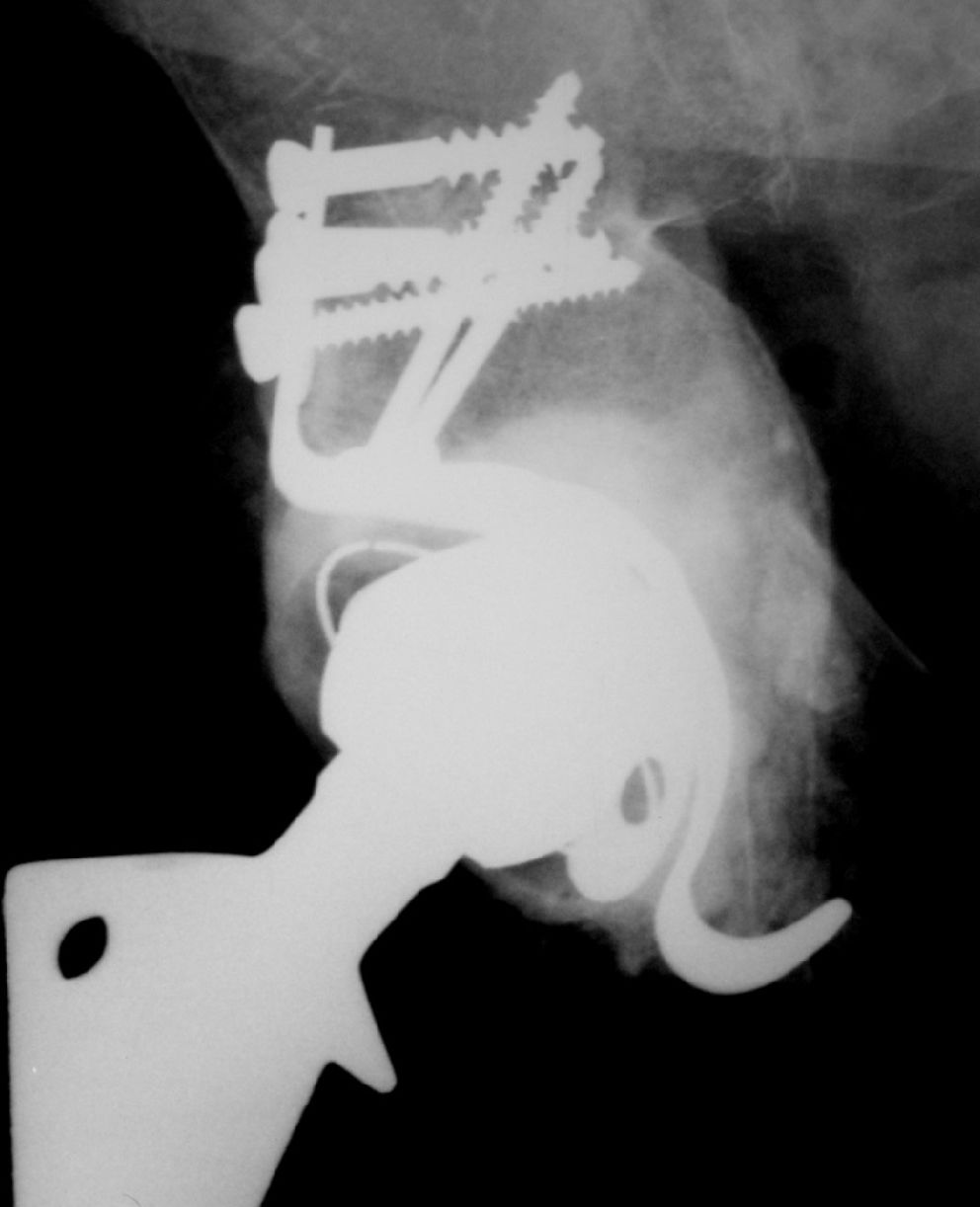
This is a cohort trial (1997–2005) of 49 patients submitted to an acetabular component revision of a total hip arthroplasty, using impacted human and bovine freeze-dried cancellous bone grafts (H&FDBG) and a reinforcement device.
OBJECTIVETo compare clinical/radiographic graft incorporation capability between cancellous bone grafts.
PATIENTS/METHODSThere were two groups: I (n=26) receiving human grafts and II (n=25) receiving bovine grafts. The average follow-up times were 55 and 49 months, respectively. Clinical analysis was based on the Merle d’Aubigné and Postel score, and the radiographic analysis involved an established score based on Conn’s et al. criteria for radiographic bone incorporation.
RESULTSNo clinical/radiographic differences were found between the groups and both showed an overall rate of 88.5% and 76% of graft incorporation (p=0.424).
CONCLUSIONThe results presented here are comparable to those in the literature with the use of deep-FG. Therefore, cancellous bone grafts can be safely and adequately used in acetabular component revision in total hip arthroplasty.
Acetabular bone loss is one of the main problems in revision total hip arthroplasty.1 Treatment of type I and II D’Antonio et al. deficiency1 with impacted cancellous morselized bone has been shown to provide good results.2 However, the treatment of severe type III and IV defects is more challenging. A valuable option in these cases is the use of bulk allografts, but the rate of failure of structural grafts that are not supported by a reinforcement device has been shown to increase over time.3 As an alternative, some authors have advocated the use of an acetabular reinforcement ring instead. This device seems to protect grafts from overstress, helping to settle the reconstructed acetabulum until the graft is integrated.4,5
Unfortunately, choosing the reconstruction technique for an acetabular defect is not the only concern. Bone grafts are also essential, and there are limited quantities of autografts to replace these losses. Moreover, the tissue requirements are far greater than the real availability of allografts.6 This situation led us to search for an alternative method of tissue processing for the disinfection and sterilization of grafts, as well as to attempt to use xenografts from a bovine source. A lyophilization process was developed for these reasons. This study was started after initial confirmation using experimental studies in animals7 and the use of this process in other general orthopedic procedures. Therefore, the aim of this study was to demonstrate the clinical and radiographic bone integration capabilities of human and bovine freeze-dried bone grafts produced at our Tissue Bank (Hospital de Clínicas de Porto Alegre - TBHCPA) in 51 consecutive acetabular reconstruction procedures.
MATERIALS AND METHODSFrom May 1, 1997 to February 1, 2005, 51 patients with severe type III and IV D’Antonio et al. acetabular defects were consecutively submitted to acetabular reconstruction in revision total hip arthroplasty (RTHA) by the hip surgery team of the Orthopedic Department of the University Hospital – HCPA. Grafts were from a human or bovine source was and were chosen at the time of the procedure according to their availability in the storeroom. Patients were divided into two groups according to the type of graft used. Group 1 (n=26) received freeze-dried grafts from a human source and Group 2 (n=25) received grafts from a bovine source.
The human bone was obtained from femoral heads. The bovine bone was obtained from Brazilian cattle, and is believed to be completely free of Bovine Spongiform Encephalopathy (BSE) infection. All bone grafts were processed at the TBHCPA, following our own processing protocol. This process allowed the bone grafts to be produced in a way that retained their major characteristics (proteins and minerals). The preparation method cannot be fully disclosed to protect intellectual property rights. In the process, proteins are denatured with 20% hydrogen peroxide, and this is followed by alcohol extraction of lipids. The end product is composed of minerals (65%) and proteins (27%), and is completely sterilized in an autoclave. The failure of the original arthroplasty was determined to be aseptic in all patients. The type and the extent of the acetabular defects had been determined from preoperative radiographs and intraoperative assessments. All patients, without exception, were followed up until March 1, 2006. Patients were fully informed of the study, which was approved by the Ethics Committee of HCPA, and a written informed consent was obtained. The clinicopathologic characteristics of patients are shown in Table 1.
Clinicopathologic characteristics of the patients.
| Features | Human Graft n = 26 (%) | Bovine Graft n = 25 (%) | P value |
|---|---|---|---|
| Female | 18 (69.2) | 20 (80.0) | 0.575 |
| Male | 8 (30.8) | 5 (20.0) | |
| Mean Age (SD) | 63.8 (14.7) | 57.7 (12.3) | 0.116 |
| Mean follow-up (Mo., SD) | 54.7 (29.08) | 49.1 (24.49) | 0.457 |
| Acetabular Deficiency | |||
| Type III | 16 (61.5) | 14 (56) | 0.907 |
| Type IV | 10 (38.5) | 11 (44) | |
The same surgeon carried out all the operations and the same reconstruction technique was used for each patient. A posterior approach was performed in all cases. The loose prosthesis was removed, and all cement, debris, granulomata and fibrous membrane were completely cleared. The acetabulum cavity was carefully reamed in order to reach the vascular bone bed, and then the acetabulum was reconstructed by cancellous morselized (chip size, approximately 8 mm3) freeze-dried bone. The chips were pressed into acetabulum defects and were carefully condensed. The flanges of the reinforcement device (MDT_, São Paulo, Brazil) were bent into shape to fit the specific anatomy of the acetabular region. The hook of the device was placed under the teardrop portion. The upper flange of the metal ring was screwed to the ilium. This should result in a stable composite, with the load-bearing host bone grafts and the implant with an impacted bone graft located beneath the ring. Afterward, a polyethylene cup was cemented into the acetabular reinforcement device. The amount of bone graft used ranged from 40 to 60 g in all cases.
Patient analysis was based upon clinical and radiographic evaluations. The clinical analyses were based on functional criteria established by Merle d’Aubigné and Postel.8
For the radiographic analyses, several subjectively established features such as radiolucency, density, trabecular bone formation and component migration were evaluated.9 A radiographic analysis of bone integration, based on the criteria of Conn et al. was thus developed to establish the bone incorporation of the grafts in the two groups. Each criterion, except migration, received an independent score ranging from 0 to 2 for each of the three zones of De Lee and Chanrley10 in the acetabulum, where 0 was the worst and 2 the best result. The sum of the scores was then, multiplied by three for the acetabulum. For migration, a score of 2, 4 or 6 was established when there was more than 6 mm, 3 to 5 mm, or less than 3 mm of prosthesis displacement, respectively. Therefore, a total of 24 points could be achieved for acetabular assessment. Adequate results were classified as those with a sum of 19 or greater.
Statistical analyses were performed using the statistical program SPSS (SPSS Inc. Chicago, IL, USA). Descriptive analyses are presented as the mean, median, standard deviation, minimum and maximum values for quantitative variables, and as percentages for qualitative variables where appropriate. For quantitative variables, the Student’s t-test was used, and in asymmetric situations, the Mann-Whitney U test was used. For the categorical variables, the Pearson’s chi-square or Fisher’s exact test was used to compare the clinical and radiographic characteristics between the two groups to test the effect of these grafts upon outcome. For assessment of possible simultaneous effects of the several factors of the clinical outcomes, bivariate analyses and Cox logistic regression were used.11 In order to compare the radiographic results and clinical outcomes, the two groups were compared by obtaining an estimate of the standardized effect-size (SES). Statistical significance was set at a 5% level (p<0.05) and a confidence interval of 95%.
RESULTSNo severe complications occurred in the early postoperative period. Only two deaths (one in each group, two and three years after the procedure) were recorded and both were unrelated to RTHA. No patients were lost to follow-up.
Overall, no minor events were observed clinically. However, in Group 1 a case of traumatic displacement three years after RTHA required surgical intervention, since attempts for clinical reduction failed. In this case, a histological analysis of the grafted region indicated there was an area of new bone formation and residual spicules from the graft material (Figure 1). In Group 2, a case of superficial infection (cellulitis) occurred six months after the procedure and was successfully treated with antibiotics. The clinical and radiographic outcomes are shown in Tables 2 and 3, respectively.
Clinical outcomes
| Clinic Evaluation Merle d’Aubigné and Postel | Human Graft (n= 26) n (%) | Bovine Graft (n=25) n (%) | P value |
|---|---|---|---|
| Very good and good | 0.384 | ||
| 12 | 8 (30.8) | 9 (36) | |
| 11 | 11 (42.3) | 10 (40) | |
| 10 | 5 (19.2) | 1 (4) | |
| Total | 24 (92.3) | 20 (80) | |
| Medium, fair and poor | |||
| 9 | 1 (3.8) | 4 (16) | |
| 8 | 1 (3.8) | 1 (4) | |
| 7 or < 7 | 0 (0) | 0 (0) | |
| Total | 2 (7.7) | 5 (20) |
Radiographic results
| Radiographic Features (SD) | Human Graft (n = 26) | Bovine Graft (n = 25) | P value |
|---|---|---|---|
| Radiolucency | 5.46 (0.84) | 5.40 (0.81) | 0.794 |
| Density | 5.52 (0.69) | 4.74 (2.04) | 0.085 |
| Trabeculation | 4.92 (1.38) | 4.32 (2.16) | 0.246 |
| Migration | 5.92 (0.39) | 5.84 (0.8) | 0.638 |
| Radiograph evaluation Scores (%) | 0.424 | ||
| Very good and good | |||
| 24 – 22 | 19 (73.1) | 16 (64) | |
| 21 – 19 | 4 (15.4) | 3 (12) | |
| Total | 23 (88.5) | 19 (76) | |
| Medium, fair and poor | |||
| 18 – 16 | 2 (7.7) | 1 (4) | |
| 15 – 13 | 1 (3.8) | 2 (8) | |
| < 13 | 0 (0) | 3(12) | |
| Total | 3 (11.5) | 6 (24) |
The problem of hip stability in aged patients is serious and has been the object of many different approaches.12–13 The goals of reconstruction of severe acetabular defects in revision arthroplasty of the hip are to restore the bone stock, to repair the hip mechanics and to obtain stability. The use of bone grafts are imperative in order to achieve these goals. Autografts are an excellent option, but the quantity of autografts is usually limited, and therefore allografts have frequently been used as an alternative. Therefore, a bone bank subject to strict quality control is necessary to minimize the risk of disease transmission. Unfortunately, there is no single technique that currently provides a solution for all deficiencies. Reconstruction with impacted cancellous morselized bone has shown to provide good results,2,14 but we are skeptical about the use of this technique in hostile acetabula, especially those of type III and IV deficiencies.1,4,5,15 Additionally, the reconstruction of structural allografts is still controversial.3 Like other authors, we also consider that the occurrence of a severe acetabular defect reconstruction should be treated with a reinforcement device combined with bone grafts. This method provides initial stability and protects grafts from mechanical stress until graft integration is achieved.4,5
For hip procedures, we believe that the most suitable grafts are those that are unaltered by processing. As assessed by physical and chemical analyses, the freeze-dried bones produced at TBHCPA retained most of their mineral and protein characteristics, and the grafts from bovine and human sources were very similar, although they did not have the same texture and malleability as their deep-frozen counterparts.16 These characteristics enabled proper handling of the freeze-dried bones after rehydration, both from a technical and mechanical viewpoint.17
By the Merle d’Aubigné and Postel criteria, the average results obtained from the human and bovine grafts were considered good and very good in 92% and 80% of the cases, respectively. Although the follow-up may be not be long enough for a more reliable clinical evaluation, it is notable that the use of human and bovine freeze-dried grafts during that period was not harmful to the patients, and there were no significant differences between the two groups. When our results were compared with studies in the literature with similar follow-up periods that used allogeneic deep-frozen grafts instead, we did not find any considerable differences that could be attributed to the use of freeze-dried grafts from bovine or human sources.4,5,15
Several studies have clinically and radiographically evaluated the use of human and bovine freeze-dried grafts in a number of bone diseases and have found that they performed well. However, few indexed articles concerning the use of human or bovine freeze-dried grafts in RTHA have been reported. This reluctance of hip surgeons to use freeze-dried grafts may also be related to the number of available grafts with different steps in the production process for distinct purposes and indications as well. As a result, different mechanical and biological responses may be obtained, leading to an unjustified concern when using this type of graft.18 Levai and Boisgard (1996) reported 30 revision cases performed on loose total hip replacements with a specific technique for acetabular reconstruction combining the use of a bovine bone substitute and a support ring. No migration of the acetabular implant or osteolysis of the heterograft was seen in 27 (90%) cases within three years after the procedure was performed. Radiologically, the heterograft gradually condensed, and its appearance was similar to that observed with allografts. In that series, two failures with implant migration and heterograft osteolysis were considered to be a result of technical bias related to the use of the Muller ring, and in both cases, the Muller ring was supported by the cancellous heterograft and not by the host bone.16 De Roeck and Drabu (2001) reported on 32 patients who underwent RTHA using cemented components and allograft impaction of processed freeze-dried bones. The overall endurance with this type of graft at a mean follow-up of four years was 91%. There were no femoral component failures, although revision was required in three patients due to failure of the acetabulum. Freeze-dried grafts may require a longer period of rehydration for adequate impaction. The results of impaction bone grafting with freeze-dried bone alone have been satisfactory, although we do not feel entirely secure with its use alone in cases of hostile acetabulum.19 Thien et al. (2001) reported an overall survival rate for acetabular reconstructions of 86% in seven cases using impacted freeze-dried cancellous bone chips and a cemented cup, with an average follow-up of seven years (range: five-nine years). At this median follow-up period, no aseptic loosening was observed and the results for freeze-dried allograft bone chips were acceptable.20 Recently, Charalambides et al., 2005, published a paper addressing their poor results in 27 RTHA cases, followed up for an average of 2.5 years, after the use of combined autograft and xenograft (Surgibone) bones. Seventeen (62%) out of the 27 patients showed apparent bone incorporation within three months. However, in three (11%) patients, there was no bone incorporation. Three other cases (11%) appeared to have what they considered a pseudoinfection (with no agent identified), and one patient, who had the revision procedure revised again suffered from a deep MRSA infection.21 Disregarding the case of unequivocal infection that was unrelated to the graft, and even considering the three cases of pseudoinfection that may not be related to the grafts, this leaves a figure of 77% success, which is similar to those figures obtained using other methods, and therefore, these results may be more acceptable than previously argued. Moreover, the authors of this paper also provided a histological sample from that patient who required revision of the replacement due to acetabular loosening, despite apparent radiographic incorporation. New bone formation from the grafted area and residual necrotic bony spicules from the graft material were observed, clearly demonstrating graft incorporation.22
Considering the radiographic criteria, and despite biases, the results obtained with human and bovine freeze-dried bone grafts from TBHCPA in this series were comparable to each other and to those previously reported in the literature, including those reports describing the use of deep-frozen allografts. Using a similar technique of impacting deep-frozen graft and cement, and with a similar follow-up, Kerboull et al. reported a similar rate of 92% of graft incorporation.4 Therefore, the success of RTHA seems to be related more to the surgeon’s skills, the inherent limitations of the techniques and the severity of the individual case, rather than to the type of graft used.
The use of freeze-dried bone grafts provides a decrease in the risk of transmission of infectious diseases and tumors, since chemical reagents including sodium hypochloride are used during processing to inactivate bacteria, viruses and probably prions.23 After processing, the bone is also sterilized,24,25 which in our tissue bank, is virtually 100% effective. Therefore, concerns related to prion transmission (EEB) with the use of freeze-dried bovine bone grafts appear to be unfounded. Also, careful selection and the country of origin of the herd (particularly Brazil, which has been always a risk-free country for EEB) should be considered.26
From a mechanical point of view, some studies of the use of non-decalcified freeze-dried bone concluded that there is no mechanical difference between freeze-dried and deep-frozen bone, and if there is one, the freeze-dried bones are favored, since they lack fat, blood and marrow cells.17,25
Although there is a shortage of data regarding xenograft use in RTHA, clinical complications have not been observed, except for those complications that are generally expected with the use of allografts or xenografts, since physical and chemical analyses have shown they are very similar. The results obtained here have shown that freeze-dried bovine bone grafts do not cause any types of adverse reactions, and therefore confirm their safety.
We conclude that processing of bones from bovine or human sources via lyophilization in our tissue bank yields bones of a suitable quality to be used in RTHA, and that the use of freeze-dried bovine bone grafts provides similar clinical and radiographic results to human freeze-dried bone grafts.