To investigate the effect of opioid receptor blockade on the myocardial protection conferred by chronic exercise and to compare exercise training with different strategies of myocardial protection (opioid infusion and brief periods of ischemia-reperfusion) preceding irreversible left anterior descending coronary ligation.
INTRODUCTION:The acute cardioprotective effects of exercise training are at least partly mediated through opioid receptor-dependent mechanisms in ischemia-reperfusion models.
METHODS:Male Wistar rats (n = 76) were randomly assigned to 7 groups: (1) control; (2) exercise training; (3) morphine; (4) intermittent ischemia-reperfusion (three alternating periods of left anterior descending coronary occlusion and reperfusion); (5) exercise training+morphine; (6) naloxone (a non-selective opioid receptor blocker) plus morphine; (7) naloxone before each exercise-training session. Myocardial infarction was established in all groups by left anterior descending coronary ligation. Exercise training was performed on a treadmill for 60 minutes, 5 times/week, for 12 weeks, at 60% peak oxygen (peak VO2). Infarct size was histologically evaluated.
RESULTS:Exercise training significantly increased exercise capacity and ΔVO2 (VO2 peak − VO2 rest) (p<0.01 vs. sedentary groups). Compared with control, all treatment groups except morphine plus naloxone and exercise training plus naloxone showed a smaller infarcted area (p<0.05). No additional decrease in infarct size occurred in the exercise training plus morphine group. No difference in myocardial capillary density (p = 0.88) was observed in any group.
CONCLUSIONS:Exercise training, morphine, exercise training plus morphine and ischemia-reperfusion groups had a smaller infarcted area than the control group. The effect of chronic exercise training in decreasing infarct size seems to occur, at least in part, through the opioid receptor stimulus and not by increasing myocardial perfusion.
Physical activity in humans is associated with improved cardiovascular health, including a reduced risk of myocardial infarction (MI) and increased survival rate following MI.1-3
A few studies have demonstrated the mechanisms of myocardial protection caused by exercise training (ET).4,5 It was discovered that exercise-induced myocardial protection is dependent on the opening of the sarcolemmal rather than the mitochondrial isoform of the myocardial KATP channel. Additionally, cardioprotection acquired through exercise is unique in that it is sustained for long periods of time, which is not true for other forms of acquired cardioprotection.6
It has been demonstrated that ET through swimming before acute MI reprogrammed the surviving myocardium, leading to an altered molecular response to MI.7 This observation might, in part, explain the protected cardiac phenotype of an exercising animal. A recent paper1 has shown that acute exercise enhances myocardial ischemic tolerance via an opioid receptor-dependent mechanism. In that study, it was also shown that exercise was associated with an early increase in myocardial mRNA levels of several opioid system genes and with sustained changes in a number of genes that regulate inflammation and apoptosis. These findings demonstrate that the acute cardioprotective effects of exercise are mediated, at least in part, through opioid receptor-dependent mechanisms that may include gene expression.
Previous studies, examining ways of protecting the heart against ischemia, reported an improved response of exercised hearts to ischemic injury. Both a single bout of exercise and long-term training, before ischemia-reperfusion (IR), reduced the infarct size and ameliorated the impaired function of rat and dog hearts.8-11 On the other hand, other studies have shown that swimming ET performed before irreversible coronary artery occlusion reduces the infarct area, even in the absence of reperfusion.12,13
In view of the effect of ET in reducing infarct size in the absence of reperfusion12,13 and the release of endorphins that occurs during ET,14-17 we tested 4 hypotheses: (1) not only ET but also opioid infusion and brief periods of IR promote myocardial protection before irreversible coronary artery occlusion; (2) blockade of the opioid system inhibits the myocardial protection conferred through chronic ET; (3) ET plus opioid infusion have additive effects in reducing the area of MI; (4) myocardial protection through different strategies occurs owing to the increase in capillary density.
METHODSAnimalsThe animal protocol was conducted according to the Guideline for the Care and Use of Laboratory Animals (NIH publication 85-23) and was approved by the institutional review board of the University of São Paulo Medical School, Brazil (No. 983/02). Our institution's ethical policy is compliant with that of the NIH. During the experimental period, rats (5 rats per cage) were fed a pellet rodent diet (Nuvital Nutrientes S/A, Curitiba, PR, Brazil) and water ad libitum in a temperature-controlled room (22°C) with a 12∶/12 h dark∶light cycle.
Male Wistar rats (8 weeks old, ∼200g) were randomly assigned to 7 groups: control (C; n = 11); ET (n = 12); morphine (M; n = 14); intermittent IR (n = 12); ET plus morphine (ET+M; n = 11); naloxone plus morphine ((M+N; n = 9); naloxone before each ET session ET+N; n = 7). All groups were submitted to MI (see details below). All sedentary animals were purchased at the same time as exercised animals and housed for the equivalent time.
ET ProtocolBefore the start of the study, rats were familiarized with a motor-driven rodent treadmill by walking at 6 m/min, no gradient, for 10 minutes, once a day, for 1 week. The exercise-trained animals were exercised 5 days/week for 12 weeks. The exercise took place at the start of the dark cycle (between 8:00 am and 10:00 am). The running speed and exercise duration were progressively increased to elicit 60% maximal oxygen uptake, for 60 minutes at the fourth week, as described previously.18 The maximal exercise test was performed at the start of the study, at the sixth week in order to adjust the training intensity and at the end of the exercise training protocol.
The exercise intensity that we chose (60% VO2max) is considered moderate intensity and it has already been shown in other studies that this intensity is sufficient to provide cardioprotection against ischemia-reperfusion injury.19
Evaluation of the ET ProtocolExercise capacity, estimated by the total distance run, was evaluated using a graded treadmill exercise protocol for rats. After adaptation to treadmill exercises over a week (10 minutes of exercise session), rats were placed in the exercise streak and allowed to acclimatize for at least 30 minutes. Exercise began at 6 m/min with no gradient and increased by 3 m/min every 3 minutes thereafter until exhaustion. The graded treadmill exercise test was performed in the trained groups at the start of the study, at the 6th week of training and after the ET period.
At the start and end of the ET protocol, peak VO2 was evaluated in the exercised groups. In the sedentary groups, these tests were done at the end of the 12-week period, 1 week after the treadmill adaptation. Oxygen consumption was determined by measuring expired gas by rapid-flow, open-circuit indirect calorimetry during a progressive running exercise test on a motor treadmill—namely, 5 m/min speed increments every 3 minutes and no gradient until exhaustion.20 Gas analysis was performed using an oxygen (S-3A/I) analyzer (Ametek, Pittsburg, PA, USA).
DrugsThe doses of drugs were based on previous studies.21 Morphine (100 μg/kg), was administered intravenously (iv) (3 periods of 5 minutes alternating with 3 periods of 5 minutes without infusion) just before the MI procedure, to the morphine and to the ET plus morphine groups. Naloxone (a non-selective opioid antagonist, 3 mg/kg, iv) plus morphine were administered to the naloxone plus morphine group, also just before the MI procedure. Naloxone (3 mg/kg, intraperitoneally ), on the other hand, was administered before each ET session, to the ET plus naloxone group.
Surgical ProcedureIn the ET groups, surgery was performed 48 h after the last ET session. In the sedentary groups, surgery was performed at the equivalent animal age. Animals were anesthetized (intraperitoneal xylazine 10 mg/kg and ketamine 90 mg/kg). An arterial cannula was inserted into the left ventricle to measure the left ventricular end-diastolic pressure (LVEDP) before and 5 minutes after the MI, and the catheter position was confirmed by the characteristic pulse pressure. The LVEDP was registered beat-to-beat (DataQ Instruments, Inc, Ohio, USA).
The rats were ventilated (Harvard respirator, 2.5 ml, 75–80 strikes/min) and the chest was opened by a left thoracotomy. The left anterior descending coronary artery was then permanently ligated with a 5/0 silk thread, close to its origin. After MI, the rats were allowed to heal for 7 days with no additional exercise given. After this additional 7 days, rats were killed and the hearts harvested.
Tissue HistologyOne week after establishing the MI, rats were anesthetized. The rats were killed at the start of the dark cycle (between 8:00 am and 10:00 am) A transverse incision below the diaphragm and bilateral thoracotomy incisions were made, and the left ventricle was cannulated with retrograde perfusion. The animal's heart was arrested in diastole by perfusion with NaCl 0.9% plus 14 mM KCl solution (pressure equal to a 13 cm water column), followed by addition of formalin for tissue fixation. Excised hearts were immersed in formalin for 24 h. The heart was then transected perpendicular to the long axis, and half of the heart was processed and embedded in Paraplast for histology. Sections (3 μm) were stained with hematoxylin–eosin for qualitative assessment, with Masson's trichrome (blue stain) for measuring the scar area, LV muscle area and thickness of the interventricular septum (IVS), and with periodic acid–Schiff for capillary density quantification. The same cross section of all hearts was used—at the mid-region of the long cardiac axis. Histomorphometric analyses were performed by the same person, who was blinded to each experimental group. Infarct size was expressed by evaluation of necrotic area, as a percentage of the region at risk (total muscle LV area). Scar area, LV muscle area and thickness of the IVS were measured by computerized planimetry (Leica Imaging Systems, Bannockburn, IL, USA). Capillary density was optically measured by a 10×10 grid superimposed on each of the 60 non-overlapping randomly selected fields at ×400 magnification within the left ventricle, involving both infarcted and non-infarcted areas.
Statistical AnalysisThe data are presented as mean ± standard deviation (SD). Analysis of variance was used for single measurements with post hoc testing by Tukey. Analysis of variance for repeated measurements with post hoc testing by Tukey was used for measurements performed before and after one procedure. The likelihood ratio was used to calculate mortality.22
Probability values <0.05 were considered statistically significant.
RESULTSMortalityNo significant difference was observed in postoperative mortality among all the groups (p = 0.99; Table 1). Most deaths occurred until 24 h after coronary ligature.
Mortality among groups (likelihood ratio).
| Group | N Total | Mortality (%) |
|---|---|---|
| C | 11 | 36.4 |
| ET | 12 | 41.7 |
| M | 14 | 35.7 |
| IR | 12 | 33.3 |
| M+N | 9 | 33.3 |
| ET+M | 11 | 27.3 |
| ET+N | 7 | 42.9 |
| p = 0.99 | ||
C = control; ET = exercise training; M = morphine; IR = intermittent ischemia-reperfusion; M+N = morphine plus naloxone; ET+M = exercise training plus morphine; ET+N = exercise training plus naloxone.
Initial body weight was similar among the groups (p = 0.94). At the end of the experiment body weight differed among the groups (p = 0.03); in the ET plus morphine group the final weight was les than that of the control group (p = 0.04). No difference in heart weights (p = 0.38) and in the ratio of heart to body weight was found among the groups (p = 0.29).
Exercise Capacity and VO2In comparison with the sedentary groups, all trained groups showed an increase in exercise capacity (Figure 1) and an increase in ΔVO2 (peak VO2 – rest VO2) after 12 weeks (ΔVO2 23.21; 24.79; 25.46 and 24.56 in groups C, IR, M+N and M, respectively; and 30.78; 30.87 and 30.01 in groups ET+M, ET+N and ET; p = 0.0001).
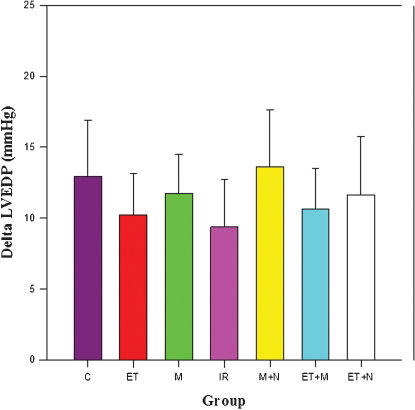
LVEDPThere was a significant increase in LVEDP 5 minutes after coronary ligation in all groups. As is shown in Figure 2, ΔLVEDP values (LVEDP 5 minutes after coronary ligation − LVEDP before coronary ligation), in mmHg, are similar, among groups (p = 0.063).
Delta left ventricular end-diastolic pressure (LVEDP) values (LVEDP 5 minutes after coronary ligation − LVEDP before coronary ligation), in mmHg, among groups. p = 0.063. C = control; ET = exercise training; M = morphine; IR = intermittent ischemia-reperfusion; M+N = morphine plus naloxone; ET+M = exercise training plus morphine; ET+N = exercise training plus naloxone.
In comparison with the control group, the treatment groups ET, M, IR and ET+M had a smaller relative scar area/LV area (0.24 vs 0.07; 0.13; 0.13; 0.12, respectively; p<0.05). This difference was not seen in the M+N and ET+N groups (relative values 0.22 and 0.37, respectively) (Figure 3). We also found that in the ET+M group there was no further decrease in infarcted area in comparison with morphine or ET alone (Figure 4). As shown in Table 3, the ET+N group had an increase in LV cross-sectional area (p<0.05) and scar area (p = 0.0001), compared with all other groups. In addition, there was an increase in IVS thickness in the ET group (compared with the control group) and in the ET+M group (compared with the control, intermittent IR and morphine groups). In the ET+N group, there was no alteration in the IVS thickness.
Infarcted area (scar area /left ventricular (LV) muscle area) among groups. *p<0.05 vs. control; §p<0.05 vs. ET+N; £p<0.05 vs ET. C = control; ET = exercise training; M = morphine; IR = intermittent ischemia-reperfusion; M+N = morphine plus naloxone; ET+M = exercise training plus morphine; ET+N = exercise training plus naloxone.
Representative transversal sections showing the scar size in different groups. Stained with Masson trichrome. The scar (rich in collagen) is shown in purple and the non-infarcted ventricle is shown in pink. (A) Control group; (B) exercise training group; (C) morphine group; (D) exercise training plus naloxone group; (E) morphine plus naloxonegroup; (F) exercise training plus morphine group; (G) intermittent ischemia-reperfusion group.
Body and heart weight.
| Group | Initial BW (g) | BW (end of study) (g) | HW (g) | HW/FBW (mg/g) |
|---|---|---|---|---|
| C | 194.3±26.3 | 499.3±40.7 | 1.64±0.2 | 3.3±0.4 |
| ET | 201.2±32 | 461.1±37.8 | 1.5±0.16 | 3.24±0.2 |
| M | 197.9±25.8 | 462.1±33.7 | 1.54±0.2 | 3.33±0.3 |
| IR | 191±22.2 | 459.3±31.6 | 1.52±0.2 | 3.29±0.3 |
| M+N | 191±13.9 | 492±21.8 | 1.68±0.1 | 3.41±0.2 |
| ET+M | 195.4±25 | 447.9±22.5* | 1.49±0.2 | 3.32±0.3 |
| ET+N | 191.6±10.7 | 449.7±19 | 1.68±0.2 | 3.72±0.5 |
Results are shown as mean±SD.
p = 0.04 vs. control
BW = body weight; HW = heart weight; HW/FBW = ratio of heart weight to final body weight; C = control; ET = exercise training; M = morphine; IR = intermittent ischemia-reperfusion; M+N = morphine plus naloxone; ET+M = exercise training plus morphine; ET+N = exercise training plus naloxone.
Histological measurements.
| Group | LV muscle area (mm2) | Scar area (mm2) | IVS thickness (mm) | Capillary density (count/mm2) |
|---|---|---|---|---|
| C | 53.6±4.6 | 12.8±5.2 | 1.8±0.4 | 150.3±50.0 |
| ET | 60.6±15.0 | 4.1±2.6‡ | 2.8±1.0§ | 146.6±38.9 |
| M | 64.2±8.1 | 8.2±3.1 | 2.2±0.6 | 168.1±50.7 |
| IR | 65.9±11.7 | 8.1±2.0 | 2.2±0.4 | 196.1±22.0 |
| M+N | 62.0±3.3 | 13.7±5.4 | 2.2±0.2 | 135.4±43.0 |
| ET+M | 65.4±11.0 | 7.5±3.2 | 3.1±0.4¶| | 136.2±46.9 |
| ET+N | 85.1±5.7* | 31.5±11.5† | 2.8±0.4 | 145.3±22.3 |
Results are shown as mean±SD.
There were no significant differences in capillary density, among the groups (Table 3; p = 0.88).
DISCUSSIONThe main findings of this study are that several strategies of myocardial protection—namely, ET, morphine and IR decreased the infarcted area (scar area/LV muscle area) in the absence of reperfusion. The effect of chronic ET in decreasing infarct size seems to occur, at least in part, through the opioid receptor stimulus since the blockade of the opioid system with naloxone annulled the reduction in infarcted area promoted by chronic ET. On the other hand, no strategy of myocardial protection promoted a significant increase in microcirculatory myocardial perfusion, as observed by the absence of alterations in the capillary density. So, our study suggests that the opioid system has an important role on the myocardial protection conferred by chronic ET, even in the absence of reperfusion.
In recent decades, convincing strategies have been developed to reduce the morbidity and mortality associated with coronary diseases, mainly by decreasing the infarcted area and consequently improving ventricular function after MI. One of the remarkable advances in this area was the discovery of reperfusion strategies.23 Other mechanisms of myocardial protection against ischemic injuries have been tested. With the exception of early reperfusion, ischemic preconditioning is one of the most powerful means of protecting the myocardium.24 So, in agreement with the pivotal study by Murry et al.,24 which demonstrated that myocardial protection against ischemic injury is abolished in the presence of prolonged ischemia, the majority of subsequent studies on this subject used a time of maximum ischemic injury of 40 minutes, followed by reperfusion.25-27 Therefore, the length of time of myocardial ischemia described in these studies did not reproduce the situation that normally occurs in patients admitted to emergency departments with acute MI.23 In the United States, for instance, fewer than 40% are treated within 90 minutes after arrival at the initial hospital as recommended by the American College of Cardiology/American Heart Association guidelines.28 Indeed, previous studies reported that the mean time between MI symptoms and arrival at the emergency department is between 2.3 and 4.7 h.28
In view of these facts and evidence of the effect of ET on reduction of the infarcted area in animals submitted to the coronary occlusion,12 our study aimed to evaluate the effect of ET, infusion of opioids, intermittent IR and the association of opioid infusion with ET on the reduction of the infarcted area after prolonged coronary occlusion. We found evidence that ET, opioid infusion and intermittent IR caused a reduction in the infarcted area after coronary occlusion, in the absence of reperfusion. In addition, this reduction was not associated with an increase in capillary density, suggesting that the cardioprotection conferred by ET was not due only to an increase in microperfusion. Moreover, we demonstrated that there is no synergic effect between opioids and ET in reduction of the infarcted area. Subsequent studies are necessary to clarify if this absence of synergic effect means that opioids and ET confer myocardial protection by stimulating the same pathway.
In our study we also noticed an increase in exercise capacity and in ΔVO2, in the trained groups. These findings corroborate previous studies.20 Additionally, an increase in the LVEDP after coronary ligation was found in all groups. This increase has already been demonstrated in others experiments,29 and indicates that an ischemic insult occurred in all groups, leading to an immediate dysfunction of the left ventricle.
To the best of our knowledge, only a few studies have described the effect of ET on cardiac performance or on the infarcted area, after an acute coronary event.30 One of these experiments,12 showed a reduction of the infarcted area, 2 days after coronary occlusion, in rats submitted to swimming for 5 weeks. Another experiment13 examined the action of ET on the infarcted area and on ventricular function after coronary occlusion in rats that had swum during 7 weeks before the MI, and were killed 4 weeks after the surgery. The rats that had swum, in comparison with sedentary and infarcted animals, had a smaller infarcted area, greater arteriolar density and modifications in the expression of genes related to the cellular metabolism, which could contribute towards the improvement of ventricular function during the process of remodeling after acute MI.
In contrast to previous studies,12,31,32 our study did not demonstrate an increase in capillary density in the treated groups, in relation to the control. This might be because, in our experiment, we submitted animals to 12 weeks of ET, and, according to a previous study,33 ET causes an increase of capillary density, in relation to the control group, after 3 weeks of training, and this increase is not seen later. After 8 weeks of training, neither an increase in capillary density nor in arteriolar density was witnessed, and after 16 weeks of training, there was an increase only in the arteriolar density, in relation to the control. So, our study was carried out in the period when an increase in capillary density caused by ET is not seen. However, if myocardial protection caused by ET does not involve an increase in capillary density, we have to ask which mechanism might cause this protection. A recent paper34 showed that swimming exercise training conducted before acute MI reprograms the surviving myocardium for altered molecular response to MI, which explains, in part, the protected cardiac phenotype of the exercised animals.
Our study has some limitations. First, we did not perform correlations between the infarcted area with the ventricular function by echocardiography. Second, we did not explore potential pathways involved in myocardial protection promoted by ET, opioids and intermittent IR, including anti-inflammatory, angiogenic factors, antioxidants or the possible impact on Toll-like receptor signaling, a critical modulator of cell survival and ischemic injury in the heart. Also, we did not have a group of animals deficient in opioid receptors, to reinforce the role of opioids in the protection conferred by ET. Additionally, to check the capillary density, we did not use specific antibodies against capillary structures, which might have made the evaluation of capillaries more difficult. We also did not have sham groups, because we considered that analysis of the data of 14 groups would be confusing.
However, our findings point to future attractive strategies to promote myocardial protection.
In conclusion, not only ET but also morphine and IR decreased the infarcted area in the absence of reperfusion. The effect of chronic ET in decreasing infarct size seems to occur, at least in part, through opioid receptor stimulus and not by the increasing microcirculatory myocardial perfusion. The demonstration that these strategies of myocardial protection effectively reduce infarct size in the absence of reperfusion, reinforces the importance of a physically active lifestyle in the protection against acute MI, and suggests that the prompt use of morphine in the emergency room may have beneficial effects on the infarcted myocardial areas, even in the absence of reperfusion.
Moreover, because ET and also other strategies of cardiovascular protection (such as infusion of opioids and intermittent IR) can lead to a reduction of the infarcted area, in the absence of reperfusion, this strengths the theory of the clinical applicability of mechanisms of cardiovascular protection against ischemia.