Gynura procumbens has been shown to decrease blood pressure via inhibition of the angiotensin-converting enzyme. However, other mechanisms that may contribute to the hypotensive effect have not been studied.
OBJECTIVES:To investigate the cardiovascular effects of a butanolic fraction of Gynura procumbens in rats.
METHODS:Anaesthetized rats were given intravenous bolus injections of butanolic fraction at doses of 2.5–20 mg/kg in vivo. The effect of butanolic fraction on vascular reactivity was recorded in isolated rat aortic rings in vitro.
RESULTS:Intravenous administrations of butanolic fraction elicited significant (p<0.001) and dose-dependent decreases in the mean arterial pressure. However, a significant (p<0.05) decrease in the heart rate was observed only at the higher doses (10 and 20 mg/kg). In isolated preparations of rat aortic rings, phenylephrine (1×10-6 M)- or potassium chloride (8×10-2 M)-precontracted endothelium-intact and -denuded tissue; butanolic fraction (1×10-6–1×10-1 g/ml) induced similar concentration-dependent relaxation of the vessels. In the presence of 2.5×10-3 and 5.0×10-3 g/ml butanolic fraction, the contractions induced by phenylephrine (1×10-9–3×10-5 M) and potassium chloride (1×10-2–8×10-2 M) were significantly antagonized. The calcium-induced vasocontractions (1×10-4–1×10-2 M) were antagonized by butanolic fraction concentration-dependently in calcium-free and high potassium (6×10-2 M) medium, as well as in calcium- and potassium-free medium containing 1×10-6 M phenylephrine. However, the contractions induced by noradrenaline (1×10-6 M) and caffeine (4.5×10-2 M) were not affected by butanolic fraction.
CONCLUSION:Butanolic fraction contains putative hypotensive compounds that appear to inhibit calcium influx via receptor-operated and/or voltage-dependent calcium channels to cause vasodilation and a consequent fall in blood pressure.
Gynura procumbens Merr. (Compositae), a fast growing herbaceous plant, is widely found in Borneo, Java, the Philippines and Peninsular Malaysia. In folk medicine, the plant is widely used for the treatment of kidney diseases, rashes and fever,1 and for hypertension.
The hypotensive activity of G. procumbens, first reported by Lam et al.,2 was shown to be partly due to inhibition of angiotensin-converting enzyme.3,4 In these studies, the effect was observed in partially purified aqueous fractions of crude ethanolic extracts of leaves. In the present study, however, attempts are made to investigate the vasorelaxant activity of another fraction that is extracted in butanol (the butanolic fraction; BU) and to elucidate the underlying mechanisms involved.
MATERIALS AND METHODSPlant MaterialWhole plants of G. procumbens, excluding the roots, were collected from the southern part of Peninsular Malaysia and authenticated at the Institute of Biological Sciences, University of Malaya. A voucher specimen (KLU 44538) was deposited in the Herbarium at the Rimba Ilmu, University of Malaya.
Extraction and Fractionation of Plant MaterialThe leaves from the fresh plant were cleaned and dried in an oven at 40 °C and then ground to powder. A crude ethanolic extract was made by macerating the powder with 96% ethanol at room temperature for 72 h. The extract was concentrated to dryness in vacuo; this resulted in a gummy residue that was then reconstituted in 80% aqueous ethanol. The resulting solution was partitioned with hexane in order to remove lipids and waxes from the preparation. The aqueous ethanolic phase was subjected to evaporation in vacuo that removed the ethanol and left an aqueous solution that contained an ethanol-soluble precipitate. This precipitate was subsequently filtered out and the precipitate-free aqueous solution was further partitioned against water-saturated n-butanol. The butanolic phase was dried in vacuo to obtain the BU, with a yield of 2.0%.
In vivo Experiments: Effect of Butanolic Fraction on the Mean Arterial Pressure and Heart Rate of Anaesthetized RatsAnimalsAdult male albino Sprague-Dawley (SD) rats, weighing between 250-300 g were obtained from the Experimental Animal Center, University of Malaya. They were kept under standard conditions and given tap water and standard rat chow ad libitum. All procedures were approved by the University of Malaya Medical Center Animal Ethics Committee.
Measurements of Blood Pressure and Heart RateThe rats were anaesthetized with sodium pentobarbitone (Rhône Mérieux Ltd, Essex, UK), 50 mg/kg, by intraperitoneal injection. The right jugular vein, left carotid artery and trachea were surgically exposed. A heparinized polyethylene cannula was inserted into the carotid artery for monitoring the pulsatile blood pressure (BP) via a pressure transducer connected to the Macintosh MacLab Set-up (AD Instruments Pty Ltd, Bella Vista, NSW, Australia). The electrocardiogram (ECG; Lead II) was recorded using needle electrodes inserted subcutaneously into the limbs of the animal to monitor heart rate (HR). The jugular vein was cannulated with heparinized polyethylene tubing for intravenous (i.v.) injections of the plant extracts. The trachea was cannulated to facilitate spontaneous respiration. The animal was kept warm with a heating lamp throughout the experiment. The set-up was allowed to equilibrate for at least 30 min before the start of the experiments.
After the equilibration period, the rats (n = 6) were injected with BU at doses of 2.5–20.0 mg/kg in a volume of 0.1 ml for each dose. BP was allowed to return to the resting level before each subsequent injection was administered. Changes in BP as a result of administration of the extracts were obtained by calculating the difference between the BP before and the lowest BP recorded after the injections. For controls, rats (n = 6) were similarly injected with an equivalent volume of the vehicle. The mean arterial pressure (MAP) was calculated from the pulsatile BP using the formula: diastolic BP + ⅓ pulse pressure.
In vitro Experiments: Studies on Isolated Rat Thoracic AortaTissue PreparationMale SD rats (250–300 g) were sacrificed by exsanguination. The descending thoracic aorta was isolated and placed in an oxygenated Krebs-Henseleit (K-H) solution of the following composition (in mM): NaCl, 118.0; KCl, 4.70; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; glucose, 11; and ascorbic acid, 0.57. The aorta was carefully cleaned of adhering fat and connective tissue and cut into 2.0–2.5 mm wide transverse rings. The aortic ring was mounted in an organ bath containing 10 ml of K-H solution by means of two parallel L-shaped stainless-steel holders inserted into the lumen. One of the holders served as an anchor while the other was connected to a force-displacement transducer to measure isometric contractile force, as recorded by a MacLab computer system (AD Instruments). The bath solution was maintained at 37 °C and bubbled continuously with a 95% O2 and CO2 gas mixture. A basal tension of 1 g was applied. Each preparation was allowed to equilibrate for 60 min with changes of bath solution every 15 min before the start of the experiments. Before each experiment, the aortic rings were stimulated at least three times with 6×10-2 M KCl until a reproducible contractile response was obtained.
For some preparations, the rings were denuded of endothelium by inserting a pair of fine forceps into the lumen of the aorta and gently rotating the aorta around the forceps. The endothelium-denuded rings were similarly equilibrated in the organ bath for 60 min. The success of endothelial denudation of the aortic rings was tested by adding acetylcholine (1×10-6 M) to the organ bath to induce relaxation in phenylephrine (PE; 1×10-6 M)-precontracted rings. A relaxation of ≥ 70% of the PE-induced contraction indicated the presence of an intact endothelial layer, whilst the lack of any relaxation indicated that the endothelium was satisfactorily removed.
Appropriate parallel control experiments (n = 6) using vehicle only were always performed concurrently in order to correct for possible changes in the sensitivity of the preparations.
Effects of Butanolic Fraction on Phenylephrine- or Potassium Chloride-precontracted RingsThe intact (n = 8) and endothelium-denuded (n = 6) aortic rings were precontracted by treatment with PE (1×10-6 M) or KCl (8×10-2 M). After the tonic responses had become stable, increasing concentrations of BU (10-6–10-1 g/ml) were added cumulatively.
Effect of Butanolic Fraction Pretreatment on Phenylephrine- or Potassium Chloride-induced ContractionsAfter equilibrium, cumulative concentration-response curves for PE (1×10-9–3×10-5 M) or KCl (1×10-2–8×10-2 M) were determined. The curves were obtained in intact (n = 6) and denuded (n = 6) rings by stepwise increase in the concentration of PE or KCl. Additions were made as soon as a steady response was obtained from the preceding concentration. After washing and recovery for 30 min in normal K-H solution, the tissue was treated with 2.5×10-3 or 5×10-3 g/ml of BU and incubated for 30 min. After the incubation period, the second cumulative concentration-response curves for PE and KCl were determined again in the presence of BU. Each preparation was exposed to only one concentration of BU.
Effect of Butanolic Fraction Pretreatment on Calcium-induced Contraction in the Presence of High PotassiumTo investigate the inhibitory effects of BU on Ca2+ influx through the voltage-dependent calcium channel (VDCC), the denuded aortic rings (n = 6) were exposed to a Ca2+-free solution in the presence of K+ (6×10-2 M) for 60 min. The Ca2+-free solution had the same composition as the normal K-H solution except that CaCl2 was omitted and ethylenediaminetetraacetic acid (EDTA) (1×10-4 M) added to ensure total elimination of extracellular Ca2+. Two successive cumulative concentration-response curves for Ca2+ (1×10-4–1×10-2 M) were obtained. The first curve was obtained in the absence of BU. The aortic rings were then washed and equilibrated for 60 min in Ca2+-free and high K+ solution. Two different concentrations of BU (2.5×10-3 or 5.0×10-3 g/ml) or vehicle were added to the bath and allowed to act for 30 min before a second cumulative concentration-response curve for Ca2+ was obtained in the presence of BU or vehicle. Each preparation was exposed to only one concentration of BU.
Effect of Butanolic Fraction Pretreatment on Calcium-induced Contraction in the Presence of PhenylephrineTo elucidate the inhibitory effects of BU on Ca2+ influx through the receptor-operated calcium channel (ROCC), the experiments were carried out in a Ca2+- and K+-free K-H solution. This solution had the same composition as the Ca2+-free K-H solution except that 1.2 mM of KH2PO4 was replaced by equimolar amount of NaH2PO4.
After equilibration, the aortic rings (n = 6) were washed with the Ca2+- and K+-free solution. Addition of PE (1×10-6 M) induced transient vasocontraction. As the PE-induced contraction reached a steady state, cumulative concentrations of Ca2+ (1×10-4–1×10-2 M) were applied in a stepwise manner to obtain the first concentration-response curve for Ca2+. The aortic rings were then washed with normal K-H solution and incubated for 45 min, after which, the medium was replaced with the Ca2+- and K+-free solution. After equilibration for 15 min, BU (2.5×10-3 or 5.0×10-3 g/ml) or vehicle were added to the bath and allowed to incubate for 30 min before the addition of PE. Cumulative concentrations of Ca2+ (1×10-4–1×10-2 M) were then added to the bath in the presence of BU or vehicle to obtain the second concentration-response curve. Each preparation was exposed to only one concentration of BU.
Effect of Butanolic Fraction Pretreatment on Noradrenaline- or Caffeine-induced Contractions in the Absence of Extracellular CalciumIn order to investigate whether the BU could interfere with the Ca2+ release from intracellular stores, the inhibiting effects of BU on noradrenaline (NA)- or caffeine-induced contractions in the absence of extracellular Ca2+ were performed.
After equilibrium in normal K-H solution, the endothelium-denuded rings (n = 6) were exposed to Ca2+-free K-H solution for 15 min, followed by the addition of NA (1×10-6 M) or caffeine (4.5×10-2 M) to induce transient contraction. The rings were then washed with normal K-H solution and incubated for 45 min to replenish the intracellular Ca2+ stores. Subsequently, the medium was replaced with Ca2+-free K-H solution. After equilibration for 15 min, the contractile responses to NA or caffeine were tested in the presence of BU (2.5×10-3 or 5.0×10-3 g/ml) or vehicle.
Chemicals and DrugsHeparin was purchased from Leo Pharmaceutical Products (Ballerup, Denmark). Drugs were obtained from Sigma Chemical Co. (St Louis, MO, USA). All drugs were dissolved in distilled water with the exception of caffeine, which was dissolved in Ca2+-free K-H solution. Other chemicals and solvents were of analytical grade.
Data AnalysisThe vasorelaxant response of BU is expressed as a percentage (%) relaxation of the PE- or KCl-induced contraction. The pIC50 value (-log IC50) is calculated as the concentration of BU (g/ml) in the bath solution required to produce a 50% reduction of the maximal contraction induced by PE or KCl.
When the cumulative concentration-response curves with PE, KCl or CaCl2 were compared, the maximal contractile response (Emax) obtained in the first response curve was taken as the 100% response and all subsequent contractions were calculated as a percentage of these values. The pEC50 value (-log EC50) was determined from the EC50 value, which is the concentration required to produce a half-effect in the concentration-response curves, calculated from individual log concentration-response curves of the PE, KCl or CaCl2 by sigmoidal non-linear regression analysis within the 95% confidence intervals using GraphPad Prism® v. 4.00 (GraphPad Software Inc., La Jolla, CA, USA).
When NA or caffeine were used as vasocontractile agonists, changes in the NA- or caffeine-induced contractions in the presence of BU or vehicle are expressed as percentages of the control agonist-induced contractions (obtained in the absence of BU or vehicle).
Statistical AnalysisAll values are expressed as mean ± SEM for n number of rats or separate experiments. Statistical differences were evaluated by analysis of variance (ANOVA) followed by Duncan's new multiple-range test and Student's t-test. A probability level of less than 0.05 (p<0.05) was considered to be significantly different. Post-hoc statistical power analysis was performed for all the experiments conducted and a value of > 0.8 was considered adequate.
RESULTSIn vivo Experiments: Effects of Butanolic Fraction on Mean Arterial Pressure and Heart Rate of RatsIntravenous administrations of BU (2.5–20 mg/kg) induced immediate and dose-dependent decreases in MAP and HR (Figure 1). BU-induced hypotension and bradycardia became significant (p<0.05) at doses of 2.5 and 10.0 mg/kg, respectively. The hypotensive effect was maximal within the first 20 to 30 s after BU treatment. Pre-dose baseline levels were fully recovered within 10 min after BU administration. The effective dose that produced a 50% reduction in the MAP (ED50) was 4.77 mg/kg of BU.
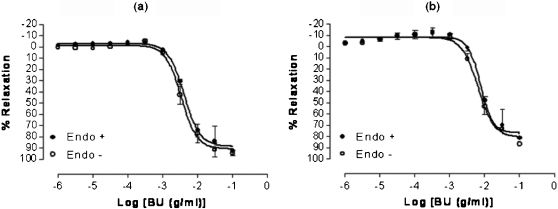
In vitro Experiments: Studies on Isolated Rat Thoracic AortaEffects of Butanolic Fraction on Phenylephrine- or Potassium Chloride-Precontracted RingsThe BU fraction at concentrations ranging from 10-6–10-1 g/ml significantly (p<0.05) inhibited the tonic contractions induced by PE and KCl in endothelial-intact and endothelial-denuded aortic rings in a concentration-dependent manner (Figure 2). The maximal relaxant responses of BU for the intact and denuded rings precontracted with PE were not significantly different (94.86 ± 3.24% and 92.76 ± 4.54%, respectively). Similarly, no differences were found in the pIC50 values for BU in intact or denuded rings (2.46 ± 0.04 and 2.38 ± 0.03, respectively).
Concentration-relaxation curves for butanolic fraction (BU) in the isolated rat aortic rings. BU-induced relaxation was studied on endothelium-intact (Endo +) and endothelium-denuded (Endo -) aortic rings precontracted with either phenylephrine (a) or KCl (b). Values are mean±S.E.M. (n = 6 - 8).
In aortic rings precontracted with KCl, there was no difference between the maximal relaxant responses of BU in intact or denuded rings (86.89 ± 8.22 and 81.66 ± 4.29%, respectively). The pIC50 values for BU in aortic ring with and without endothelium were also not significantly different (2.11 ± 0.06 and 2.19 ± 0.07, respectively).
The pIC50 values of BU in PE-precontracted aortic rings were significantly (p<0.05) higher than that in KCl-precontracted rings for both intact and denuded preparations.
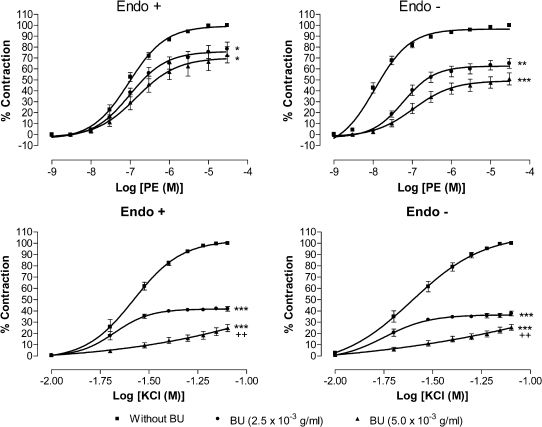
Effect of Butanolic Fraction Pretreatment on Phenylephrine- or Potassium Chloride-induced ContractionsPretreatments with BU at 2.5×10-3 and 5.0×10-3 g/ml for 30 min potently (p<0.05) inhibited the PE-induced vasocontraction in a concentration-dependent manner and shifted the concentration-response curve toward the right in a non-parallel manner with decreased Emax values in both endothelium-intact and -denuded aortic preparations (Figure 3). Table 1 shows the effect of BU on the Emax and pEC50 values for PE and KCl in endothelium-intact and endothelium-denuded aortic rings. Although BU did not alter the pEC50 value for PE in endothelium-intact rings, it caused a significant (p<0.05) reduction in the pEC50 value in the denuded rings.
Effect of butanolic fraction (BU) on phenylephrine and KCl-induced contraction. Concentration-response curves for both agonists were determined in endothelium-intact (Endo +) and endothelium-denuded (Endo -) aortic rings in the absence (without BU) and presence of BU (2.5×10-3 or 5.0×10-3 g/ml). Values are mean±S.E.M. (n = 6). *p<0.05; **p<0.01 and ***p<0.001 compared with controls (without BU).
Effect of butanolic fraction (BU) on the Emax and pEC50 values for phenylephrine and KCl in endothelium-intact (Endo +) or endothelium-denuded (Endo -) aortic rings.
| Emax (%) | pEC50 | |||
|---|---|---|---|---|
| BU (× 10-3 g/ml) | Endo + | Endo - | Endo + | Endo - |
| Phenylephrine | ||||
| 0 | 100 | 100 | 7.11 ± 0.06 | 7.88 ± 0.14 |
| 2.5 | 78.35 ± 6.27* | 65.17 ± 4.55** | 7.01 ± 0.11 | 7.23 ± 0.11* |
| 5.0 | 72.93 ± 7.43* | 50.51 ± 5.75** | 6.86 ± 0.21 | 6.96 ± 0.13* |
| KCl | ||||
| 0 | 100 | 100 | 1.57 ± 0.01 | 1.59 ± 0.03 |
| 2.5 | 41.81 ± 2.01*** | 38.42 ± 2.21*** | 1.65 ± 0.04 | 1.77 ± 0.09 |
| 5.0 | 24.81 ± 3.21***++ | 25.43 ± 2.66 ***++ | 1.42 ± 0.44* | 1.46 ± 0.06* |
Values are means ± S.E.M. (n = 6).
*P < 0.05; ** P < 0.01 and ***P < 0.001 compared with the control group
Similarly, pretreatment with BU suppressed (p<0.05) the cumulative concentration contraction induced by KCl in a concentration-dependent manner. The Emax values for KCl in endothelium-intact and endothelium-denuded aortic rings were significantly (p<0.05) depressed in the presence of BU. There was a reduction of pEC50 values for KCl in the presence of BU at 5.0×10-3 g/ml in both intact and denuded rings.
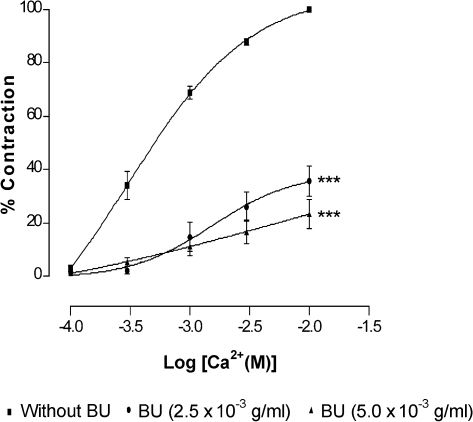
Effect of Butanolic Fraction Pretreatment on Calcium-induced Contraction in the Presence of High PotassiumAs shown in Figure 4, pretreatment of BU attenuated the CaCl2-induced contraction of denuded rat aorta exposed to Ca2+-free medium containing high K+. Preincubation of the rings with BU at 2.5×10-3 and 5.0×10-3 g/ml significantly (p<0.001) reduced the Emax values for CaCl2 in a concentration-dependent manner (Table 2). Similarly, the pEC50 values for CaCl2 were reduced (p<0.001) in the presence of BU.
Effect of butanolic fraction (BU) on CaCl2-induced contraction in Ca2+-free solution containing high K+ (60 mM). Concentration-response curves for CaCl2 were determined in endothelium-denuded aortic rings in the absence (without BU) and presence of BU (2.5×10-3 or 5.0×10-3 g/ml). Values are mean±S.E.M. (n = 6). ***p<0.001 compared with controls (without BU).
Effect of butanolic fraction (BU) on the Emax and pEC50 values for CaCl2 in endothelium-denuded aortic rings in Ca2+-free solution containing high K+.
| BU (× 10-3 g/ml) | Emax (%) | pEC50 |
|---|---|---|
| 0 | 100 | 3.22 ± 0.03 |
| 2.5 | 35.68 ± 5.69*** | 2.83 ± 0.09* |
| 5.0 | 23.37 ± 5.31*** | 2.93 ± 0.17* |
Values are means± S.E.M. (n = 6).
*P<0.05 and ***P<0.001 compared with the control group
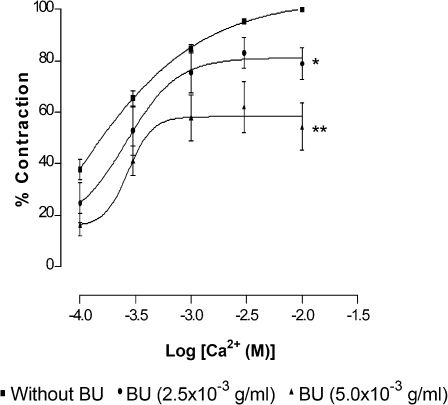
In Ca2+- and K+-free solution, after PE induced a stable aortic contraction, Ca2+ at increasing concentrations (1×10-4–1×10-2 M) were cumulatively added to the organ bath. A sustained contraction was generated, which increased with the concentration of Ca2+. Preincubation of the aortic rings with BU (2.5×10-3 and 5.0×10-3 g/ml) for 15 min before PE application significantly (p<0.05) inhibited the Ca2+-induced contraction in a concentration-dependent manner (Figure 5). BU significantly (p<0.05) suppressed the Emax values of CaCl2 without altering the pEC50 values (Table 3).
Effect of butanolic fraction (BU) on CaCl2-induced contraction in Ca2+- and K+- free solution containing phenylephrine (PE). Concentration-response curves for CaCl2 were determined in endothelium-denuded aortic rings in the absence (without BU) and presence of BU (2.5×10-3 or 5.0×10-3 g/ml). Values are mean±S.E.M. (n = 6). *p<0.05; and **p<0.01 compared with controls (without BU).
Effect of butanolic fraction (BU) on the Emax and pEC50 values for CaCl2 in endothelium-denuded aortic rings in Ca2+- and K+- free solution containing phenylephrine.
| BU (× 10-3 g/ml) | Emax (%) | pEC50 |
|---|---|---|
| 0 | 100 | 3.37 ± 0.07 |
| 2.5 | 71.93 ± 5.55* | 3.47 ± 0.06 |
| 5.0 | 53.78 ± 5.31** | 5.34 ± 0.09 |
Values are means± S.E.M. (n = 6).
*P<0.05 and **P<0.01 compared with the control group
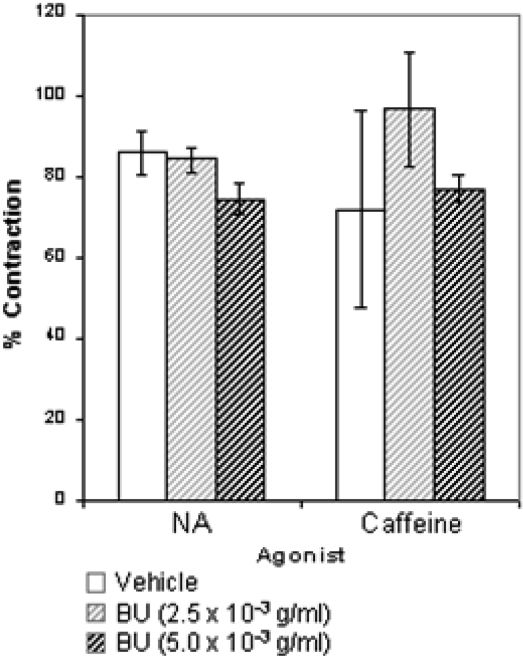
The results presented in Figure 6 show that the contractions induced by either NA or caffeine in a Ca2+-free solution were not significantly affected by the addition of BU.
Effect of butanolic fraction (BU)on noradrenaline- and caffeine-induced contraction in endothelium-denuded aortic rings in Ca2+-free solution. Aortic rings were preincubated with vehicle (control) or BU (2.5×10-3 or 5.0×10-3 g/ml) and noradrenaline (10-6 M) or caffeine (45 mM) was added to trigger the contractions. Values are mean±S.E.M. (n = 6).
The results (Figure 1) show that BU obtained from the crude ethanolic extract of G. procumbens may contain compounds that appear to exert a strong, dose-dependent and immediate BP-lowering effect in rats, with the maximal response being reached within approximately 20 s of administration. The duration of the hypotensive effect seems to last about 10 min. Significant bradycardia was also observed at the higher doses (10.0 and 20.0 gm/kg) of BU, which may contribute to the hypotensive effect.
However, the hypotensive effect could also be brought about by putative compounds present in BU that act by reducing the resistance of peripheral blood vessels. In in vitro studies, BU could cause relaxation in endothelium-intact and endothelium-denuded aortic rings precontracted by PE or KCl, suggesting that vasorelaxation could be a basis for the hypotensive action (Figure 2).
Vascular endothelium plays an important role in controlling vascular tone through the secretion of both relaxant and contractile factors. Endothelial cells respond to chemical and physical stimulation by producing several potent vasodilator substances such as bradykinin, prostaglandins, in the form of prostacyclin,5,6 and nitric oxide.7 However, data presented here seem to suggest that the action of BU is mediated via a direct effect on the vascular smooth muscle (VSM) and not through the endothelium. This is a result of the finding (Figure 2) that relaxation brought about by BU persisted in both the endothelium-intact and endothelium-denuded aortic rings, indicating that the endothelium may not be involved in BU-induced vasorelaxation.
Activation of α1-adrenoceptors on VSM by PE induces smooth muscle contraction,8 while specific α1-adrenoceptor antagonists such as prazosin competitively antagonise the contractile responses to PE by shifting parallel to the right of the PE concentration-response curve without modifying the maximal responses.9,10 In this study, however, the BU fraction at 2.5×10-3 and 5.0×10-3 g/ml was able not only to cause a right- and down-shift of the PE concentration-response curve but also to reduce the maximal response (Figure 3). This suggests that BU acts as a non-competitive antagonist against the PE-induced contraction. Therefore, BU has no specific antagonistic effect on α1-adrenoceptors and the vasorelaxant effect of BU could not have been mediated directly via the α1-adrenoceptors.
Vascular smooth muscle contraction is triggered by a rise in free cytoplasmic Ca2+ level brought about by the opening of VDCCs and ROCCs11,12 located on cell membranes. These channels can be activated by high extracellular K+ for VDCC11 and PE for ROCC,12 but by different mechanisms of the downstream signalling pathways.13-15 In the present study, the data (Figure 2) obtained indicated that BU is able to exert vasorelaxation in the endothelium-intact and endothelium-denuded aortic rings precontracted with PE or high K+ in a concentration-dependent manner. In addition, BU was also able to concentration-dependently antagonise the contractions induced by PE or KCl in aortic rings with or without endothelium (Figure 3). These observations suggest that BU may interfere with the Ca2+ channels of the VSM, resulting in a decrease in the cytoplasmic Ca2+ levels and, hence, in the contraction. Indeed, the results of further investigations (Figure 4), wherein the aortic rings were incubated in the presence of BU in a medium that is Ca2+-free but with high K+ or in another that is both Ca2+- and K+-free and containing PE (Figure 5), reveal that BU appears to bring about relaxation of the VSM by blocking both the VOCC and ROCC.
The rise in free cytoplasmic Ca2+ level that is necessary for VSM contraction to occur can also be brought about by activation of the ryanodine receptor located on sarcoplasmic reticulum (SR)16,17 by a spike in cytoplasmic Ca2+ (the calcium-induced calcium release mechanism).18-20 In addition, activation of the inositol trisphosphate (IP3) receptor located on the SR can increase the cytoplasmic Ca2+ level as well. These receptors are sensitive to caffeine for ryanodine16,21 and NA for IP3.22,23 In the present study, the influence of BU on Ca2+ release from intracellular stores sensitive to NA and caffeine was analyzed, wherein it was found that BU did not alter the vasocontraction induced by NA or by caffeine (Figure 6). Thus, it seems unlikely that the vasorelaxant effect of BU could involve the reduction of Ca2+ release from intracellular stores via inhibition of IP3 or ryanodine receptors.
Another possible mechanism that leads to vasorelaxation is the opening of potassium channels which would increase the potassium efflux, leading to membrane repolarization and/or hyperpolarization.16,24,25 This effect subsequently lowers the opening probability of VDCC,26 restrains agonist-induced Ca2+ release from intracellular stores through inhibition of IP3 formation,27 decreases the sensitivity of intracellular contractile elements to Ca2+,28 and accelerates the clearance of intracellular Ca2+ via the Na+/Ca2+ exchanger.24,25,29 In the current study, BU was shown to inhibit the Ca2+ influx through VDCC and slightly decrease, although not significantly, the NA-induced Ca2+ release via IP3 receptors. These observations could be as a result of the potassium channel-opening property of BU. However, this postulation needs to be confirmed by further investigations.
CONCLUSIONThis study conclusively demonstrates that the leaves of G. procumbens contain putative principles that exhibit a BP-lowering effect in rats in both in vivo and in vitro studies. The BP-lowering effect of BU appears to be as a result of vasodilatation through inhibition of the Ca2+ influx via the VDCC and ROCC.
This work was partially funded by the Intensified Research Priority Areas (IRPA) Programme of the Ministry of Science, Technology and Environment, Malaysia (Project No. 06-04-01-0115) and Vote F from University of Malaya.