This study proposes a new approach that considers uncertainty in predicting and quantifying the presence and severity of diabetic peripheral neuropathy.
METHODS:A rule-based fuzzy expert system was designed by four experts in diabetic neuropathy. The model variables were used to classify neuropathy in diabetic patients, defining it as mild, moderate, or severe. System performance was evaluated by means of the Kappa agreement measure, comparing the results of the model with those generated by the experts in an assessment of 50 patients. Accuracy was evaluated by an ROC curve analysis obtained based on 50 other cases; the results of those clinical assessments were considered to be the gold standard.
RESULTS:According to the Kappa analysis, the model was in moderate agreement with expert opinions. The ROC analysis (evaluation of accuracy) determined an area under the curve equal to 0.91, demonstrating very good consistency in classifying patients with diabetic neuropathy.
CONCLUSION:The model efficiently classified diabetic patients with different degrees of neuropathy severity. In addition, the model provides a way to quantify diabetic neuropathy severity and allows a more accurate patient condition assessment.
Diabetic sensorimotor polyneuropathy (DSPN) is the most common chronic complication associated with diabetes mellitus. DSPN encompasses a group of clinical and subclinical syndromes with varied etiologies and clinical and laboratorial manifestations, defined by the progressive diffuse or focal degeneration of peripheral somatic and autonomic nerve fibers (1). Its prevalence is directly related to diabetes duration, patient age, and metabolic control. Approximately 20% of diabetic patients will develop clinically significant neuropathy within 10 years of diabetes onset, and this proportion can increase to 50% after 10 or 15 years (2). Somatosensory inputs decrease as diabetic neuropathy advances, and motor output becomes progressively more impaired. These issues may lead to the higher instability and kinematic abnormalities observed during locomotion and static posture in diabetic neuropathic patients (3–5). Sensory, motor, and autonomic deficits may increase the risk of falling and ulcer formation in these patients (6,7).
The diagnosis of DSPN is generally made based on neurologic signs and symptoms and electrophysiologic measurements (8). However, health experts define the degree of neuropathy in a subjective way. Because neuropathy onset is insidious and manifests differently for each patient, expert experience plays an important role in the classification of neuropathy.
Many screening instruments with numerous composite scores are used to evaluate neuropathy. The tools that are most frequently used in the literature are the Michigan Neuropathy Screening Instrument questionnaire (MNSIq) and Physical Assessment (9) and the Diabetic Neuropathy Symptom (DNS) score, both of which are used solely for screening (10). Neither instrument provides a disease severity rating. Other instruments, such as the Neuropathy Disability Score (NDS) (11), Neuropathy Impairment Score (NIS) (12), Toronto Clinical Scoring System (TCSS) (13), and Clinical Neuropathy Examination (CNE) (14,15), assign patients to different disease levels.
Although these questionnaires are widely used in clinical practice, there is no consensus regarding which tool is best suited for disease diagnosis or severity evaluation. Thus, it is common for individual clinics to develop their own protocols, in which specialized laboratory tests are used in conjunction with questionnaires (16).
The gold standard for detecting DSPN is the nerve conduction velocity test. It can diagnose sensory and motor losses due to neuropathy even when dysfunction is subclinical, and it can predict ulceration and mortality in diabetic patients (8,17,18). However, this invasive, painful test must be performed by specialists, and it is not commonly available in public health units.
The 10-g monofilament evaluation is another specialized test that is strongly recommended by the International Consensus on the Diabetic Foot. Monofilament evaluation is a good tool to assess the loss of protective sensation related to diabetic neuropathy (19). It has a high reproducibility and specificity and may be used to predict ulceration and amputation risks (20).
Glycemic control may be considered as an auxiliary measure for predicting chronic diabetes mellitus complications, including DSPN. However, this information is questionable because daily glycemic rates are highly variable. The clinical measurement of glycosylated hemoglobin (HbA1c) is more recommended for diabetes management. Maintaining HbA1c levels below 6.5% is a major goal of diabetes management because A1c levels correlate well with diabetes complications risks (21).
The tests considered here involve some uncertainties with respect to the measurement process and subsequent diagnosis. Furthermore, the boundary between sickness and health is not always clear. When this boundary is defined, the classification of severity follows binary logic, ignoring the fact that complications can represent continuous processes. Furthermore, severity classification depends on a subjective analysis by the examiner. It is important to consider these uncertainties to obtain a precise classification of DSPN severity. One of the most powerful tools to deal with this type of data is fuzzy set theory.
Fuzzy set theory was introduced by Lotfi Zadeh in 1965. It is a mathematical entity that was developed to handle problems in which there is uncertainty about whether an element is part of a certain set. Fuzzy sets structure makes it possible to deal with the concept of partial truths and the elaboration of linguistic variables (22). Thus, this theory allows us to assess the degree of diabetic neuropathy.
In this paper, we propose a fuzzy expert-based rule system for quantifying diabetic neuropathy to categorize patients into severity subsets.
METHODSData acquisitionThe dataset was composed of 50 real cases from the Hospital of the University of São Paulo (Ethics Committee protocol 0305/08).
The inclusion criteria were: diagnosis of type II diabetes; less than 65 years old; no lower limb amputation or severe orthopedic foot deformities (e.g., Charcot arthropathy) confirmed by radiography; no peripheral or central vestibulopathy or any other neurological disease unrelated to diabetes. These criteria were verified by a staff physician from the University Hospital.
Model developmentFuzzy rule-based models have a simple structure and consist of four major components: 1) a fuzzification module, which translates crisp inputs (classical measurements) into fuzzy values through linguistic variables; 2) an if-then fuzzy rule base, which consists of a set of conditioned fuzzy propositions; 3) an inference method, which applies fuzzy reasoning mechanisms to obtain outputs (i.e., carries out the computation using fuzzy rules); and 4) a defuzzification module, which translates fuzzy outputs back to crisp values, if necessary (22).
The fuzzy model was elaborated based on the experience and knowledge of four diabetic neuropathy experts. These experts considered the following inputs as the most important variables: symptom assessment based on the MNSI questionnaire score, sign examination based on the MNSI Physical Assessment score, level of HbA1c, and the duration of diabetes, measured in years. The chosen tests require only simple procedures and can thus be applied in a clinical environment without the use of sophisticated equipment or specialized personnel.
The experts defined limiting values to establish each fuzzy set. They considered the impact of each assessment item on disease degree based on specific literature, their own daily experience in diagnosing these patients and the simplicity of the screening method. For the MNSI questionnaire, items that assess nocturnal worsening of symptoms and past ulcers were weighted as 2.0 points, while questions about numbness, burning, tingling, pins and the diagnosis of neuropathy from another health professional were weighted as 1.0 point. The physical evaluation of the MNSI foot inspection scored 0.5 point, and the monofilament and vibration perception tests scored 1.0 point each. Each foot was evaluated separately.
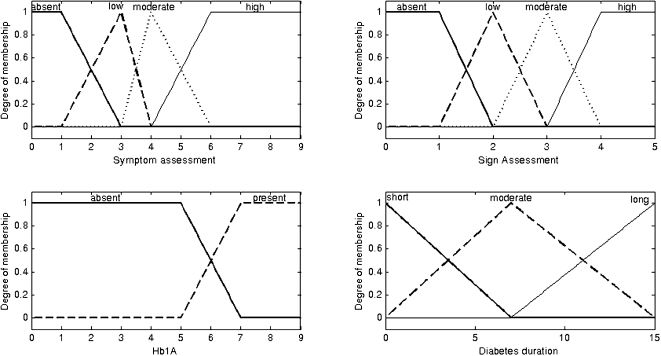
The input variables were fuzzified using the following linguistic terms:
- (a)
Symptom assessment (score): (1) absent, (2) low, (3) moderate, or (4) high;
- (b)
Sign assessment (score): (1) absent, (2) low, (3) moderate, or (4) high;
- (c)
HbA1c levels: (1) absent or (2) present;
- (d)
Diabetes duration (measured in years): (1) short, (2) moderate, or (3) long.
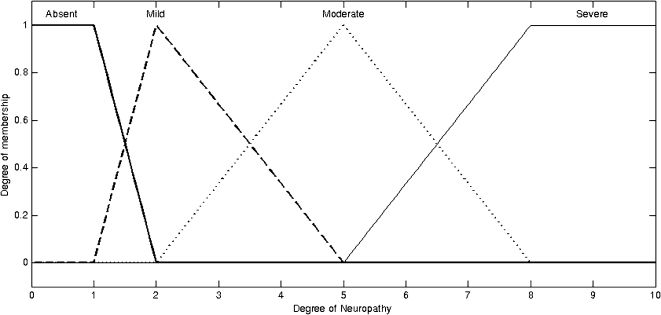
The fuzzy output sets, shown in Figure 1, indicate the degree of neuropathy. The sets were validated by expert opinion using the following linguistic terms: absent neuropathy, mild neuropathy, moderate neuropathy, and severe neuropathy (Figure 2).
Based on the fuzzy input sets, linguistic rules were elaborated using a combinatory analysis of those variables. The consequence of each fuzzy rule was determined by the experts, and 96 rules were elaborated in the form of the following example:
“IF symptom assessment is highAND sign assessment is moderateAND HbA1c is presentAND diabetes duration is longTHEN neuropathy is severe”.
Using this set of rules and the system input and output values, it is possible to assess the level of diabetic neuropathy of any patient from which those input variables were measured. Neuropathy severity was assessed by means of a Mamdani inference process, and the Center of Area defuzzification method was applied. This produces a quantitative value for the neuropathy level, defined as a number between 0 and 10.
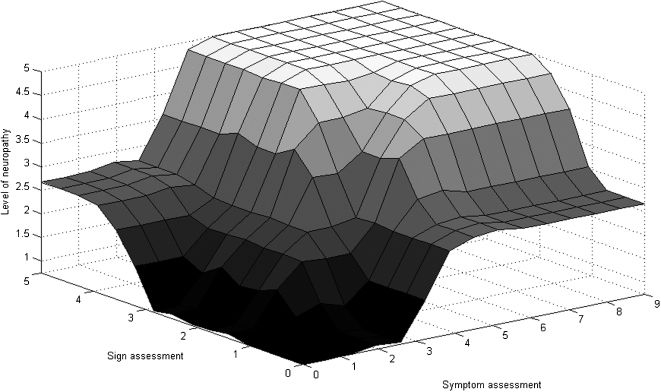
We used a dataset comprising 50 hypothetical cases of diabetic neuropathy elaborated by the experts to refine the fuzzy model. To illustrate the mathematical function determined by the rule base of the model, Figure 3 shows the surface representing the severity of the disease based on the inputted signs and symptoms.
Performance evaluationWe evaluated the model performance in two different ways: i) we assessed the agreement between the model and expert classifications with the Kappa test; and ii) we evaluated the model sensitivity and specificity with an ROC analysis, which is considered the gold standard for neuropathy diagnosis.
For the agreement analysis, four experts evaluated a separate set of 50 real cases based on clinical data gathered from patient charts. Each case received a linguistic classification indicating the degree of neuropathy (absent, mild, moderate, or severe). The model produces numerical values, which were sorted into the following classes: (i) 0 – 2.5: absent neuropathy; (ii) 2.6 – 4.5: mild neuropathy; (iii) 4.6 – 7.5: moderate neuropathy; and (iv) 7.6 – 10.0: severe neuropathy.
The model results were compared with those provided by each expert, and the expert analyses were compared with each other. Thus, we were able to determine whether model performance was consistent with human expert assessments.
RESULTSTable 1 shows the Kappa values for test agreement between the model and the experts. The level of agreement among experts was only moderate (23), highlighting the uncertainty in classifying the degree of diabetic neuropathy in these patients. The agreement between the model and the experts was also moderate, i.e., the model agrees with the experts as much as they agree with each other. In all statistical tests, p<0.001.
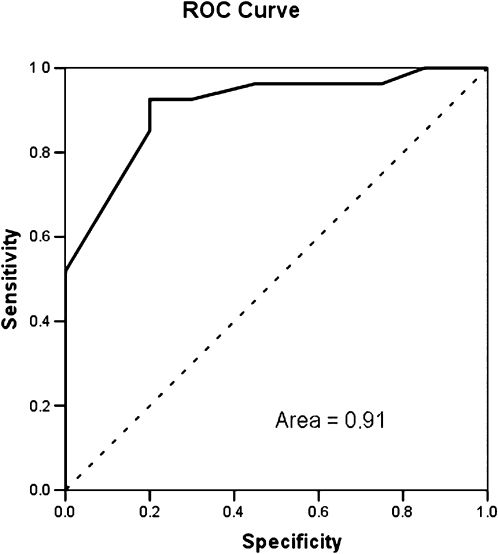
Figure 4 shows the ROC performance of the model. The area under the curve was 0.91, indicating that the model is able to identify neuropathy in diabetic patients.
DISCUSSIONThere is no doubt that the process of identifying and classifying diabetic patients as neuropathic is unclear and subjective. Uncertainties in both symptom measurement and diagnoses complicate this process. The skill and experience of the expert evaluating the patient and the available instruments are important factors. Thus, each clinic sets its own protocols without an established consensus.
Bus et al. (24) identified neuropathy based solely on the 10-g monofilament test or vibratory sensitivity. Allet et al. (25) categorized neuropathy using only the vibratory sensitivity test. However, the isolated use of different identification and classification methods can lead to bias because mild neuropathies often cannot be identified.
Moreover, symptoms may vary from patient to patient, especially in the early stages of the disease. Although the disease is symmetrical and distal, there can be differences in the plantar areas affected by sensory impairments, the type of foot deformity (cavus, planus, hammer toes, etc.) and the extent of motor impairment. This means that one patient may have clinical features that cannot be compared directly to those of another patient, even if both patients were diagnosed with the same degree of neuropathy.
Few studies have proposed the combination of several techniques to identify diabetic neuropathy. For example, Bacarin et al. (26) suggested the use of the MNSIq, 10-g monofilament sensitivity test, and disease duration as criteria for neuropathic severity. However, this proposal does not take the vagueness of the diagnostic process into account.
Few studies have sought to address these problems. Duckstein et al. used fuzzy set structure to classify diabetic neuropathy based on an invasive electrophysiological test (27). However, this equipment requires specific knowledge and appropriate professional training, and it is thus not widely used in public health systems, especially in poorer countries. Furthermore, although this test is very reliable, it cannot detect neuropathy in the early stages of the disease. Indeed, the electrophysiological test only provides clinically useful information when neural degeneration has already begun.
Conversely, the fuzzy model proposed here was based on expert knowledge and four simple variables that are easily determined in routine clinical assessments, avoiding the necessity of sophisticated and invasive methods that require a specially trained staff. The model was able to address diagnostic and inter-measurement uncertainties, identify neuropathy, and quantify its severity.
The agreement analysis presented here showed that this model is able to classify the severity of the disease in the same manner as human experts. This suggests that the system would pass the Turing test. The moderate agreement among the interviewed experts shows how difficult it is to come to consensus on the clinical severity of neuropathy based solely on physical anamnesis. Therefore, fuzzy theory can be used to address this problem.
One of the most important contributions of this model is its ability to detect diabetic neuropathy early in its progression. The literature suggests that diabetic neuropathy may be irreversible (2); therefore, timely preventive intervention at early stages may help to ensure a better quality of life for diabetic patients.
This paper proposes a fuzzy rule-based model to create an expert system to support the classification of diabetic neuropathy into different levels based on severity. The model showed an adequate level of agreement with the expert classifications, as well as high assessment accuracy in real patients. The model is able to classify patients with different degrees of neuropathy, which may improve treatment effectiveness and aid health professionals in managing this syndrome. This system supports the concept of classifying patients with different degrees of diabetic neuropathy, rather than identifying them as simply neuropathic or non-neuropathic.
This model is able to match the ability of clinical experts in classifying neuropathy severity in diabetic patients, and it does not require sophisticated testing or equipment. This model can be implemented in any public health system and may serve as an important instrument for preventing complications due to disease progression. The model also enables immediate intervention in early stages of neuropathy to maintain patient quality of life.
AUTHOR CONTRIBUTIONSAll the authors participated in the study design, results discussion, and final revision. Picon AP and Ortega NRS wrote the first draft and were responsible for fuzzy model development and statistical analysis. Sartor C and Watari R acquired patient data at the hospital. Sacco ICN and Sartor C coordinated the research and participated as experts in fuzzy model development.
We would like to acknowledge funding from CNPq (Picon PhD scholarship 140848/2009-6, Watari Master scholarship 556374/2010-0) and CAPES (Sartor scholarship). We would also like to thank Aline Arcanjo Gomes and Tatiana de Almeida Bacarin for their participation as experts in the development of the fuzzy model.
No potential conflict of interest was reported.