To describe the morphological features of atherosclerosis in the aortas of autopsied patients (ranging from young adults to the elderly), thus providing new tools for a more sensitive morphological evaluation.
METHOD:We collected 141 aorta samples. We assessed the macroscopic degree of atherosclerosis, thickness of the intima and media, lipid and collagen depositions in the intima, and the infiltration of mast cells into the layers of the aorta. We correlated the findings with gender, age, race and cause of death.
RESULTS:The degree of atherosclerosis was significantly higher in the elderly. The aorta was thicker in the elderly and in cases with a cardiovascular cause of death. The thickness of the intima was significantly greater in the elderly, in males and in cases with a cardiovascular cause of death. The lipid content in the intima of the aorta was significantly higher in Caucasians. Older people and men had a significantly higher number of mast cells.
CONCLUSION:A macroscopic evaluation is a good indicator of the severity of atherosclerosis, but a more detailed analysis, namely evaluating the thickness of the layers of the aorta and the number of mast cells, may further elucidate the changes in the constituents of this vessel.
Several factors are related to the pathogenesis of atherosclerosis, including genetic predisposition, local changes such as an increase in endothelial permeability, toxic inflammatory processes, lifestyle and eating habits, and a large number of mast cells in areas of advanced atherosclerotic lesions (1,2).
Approximately 10% of the world’s population has clinical evidence of atherosclerosis. Atherosclerosis does not usually cause death but is responsible for various cardiovascular diseases. Although cardiovascular diseases caused less than 10% of deaths worldwide at the beginning of the 20th century, they were responsible for approximately 50% of all deaths in developed countries and 25% in developing countries at the beginning of the twenty-first century (3,4). In 2000, ischemic heart disease was estimated to be the leading cause of death worldwide, and cerebrovascular disease was estimated to be the third (5). Cardiovascular diseases are the leading cause of death in Brazil and are responsible for approximately 32% of deaths in all adult age groups (6).
The characteristics of the lesion are more relevant than its size when determining the severity of the atherosclerotic lesion (7). Although advanced lesions may produce symptoms, the preceding stages are clinically silent (8). Given the high prevalence of atherosclerosis, its complications and its slow development with early lesions that may appear in childhood and develop gradually over time (9), most evolutionary studies have used test animals exposed to risk factors (10,11). Moreover, very few autopsy studies address the morphological development of atherosclerosis in humans (12). Thus, the aim of the present study is to describe the morphological features of atherosclerosis in the aortas of autopsied patients from young adults to the elderly, thereby providing new tools for a more sensitive morphological evaluation and epidemiological data for the development of preventive approaches to atherosclerosis. Our studies demonstrate that the aortas of elderly patients and patients with cardiovascular-related death were thicker and that there was a significant, positive correlation between thickness and age. These data are in accordance with the grading analysis findings performed by macroscopic examination.
METHODSThis study was approved by the Research Ethics Committee under protocol II04/08. A total of 141 aorta segments were obtained from 141 subjects autopsied at Hospital das Clínicas of Universidade Federal do Triângulo Mineiro from 1963 to 2008. The patients ranged from 18 to 85 years of age and were included regardless of the cause of death or underlying disease. Variables such as age, gender, ethnicity, and cause of death were obtained from the autopsy reports. The cases were classified into age groups of 10-year intervals. The patients who were 60 years or older were considered elderly, and the remaining patients were classified as non-elderly. The autopsied patients were divided into Caucasian and non-Caucasian groups.
Degree of atherosclerosis: The selected aortas were 15 cm long or more. The degree of atherosclerosis was evaluated based on the intensity of fibrosis and calcification observed. Taking these criteria into account, three researchers individually and subjectively described the degree of atherosclerosis, with one scale unnumbered, measuring 12.0 cm. In this scale, each researcher recorded a point corresponding to the degree of atherosclerosis. The distance from 0.0 cm to the point on the scale was measured with a ruler, being established specific numbers for each degree of atherosclerosis. The mean distance was used to represent the degree of atherosclerosis for each aorta analyzed.
Thickness of the tunica intima and tunica media of the aorta: After the macroscopic analysis, the aorta segments were fixed in formaldehyde. Then, the segments were stained with Verhoeff’s stain to quantify the thickness of the tunica intima and tunica media of the aorta (13). Forty measurements of the tunica intima and thirty measurements of the tunica media were obtained after the calculation of the cumulative mean test (14). The measurements were performed using a 5X-objective. The thickness of the tunica intima was measured from the endothelial layer to the internal elastic lamina, and the thickness of the tunica media was measured from the internal elastic lamina to the external elastic lamina. We obtained five measurements per field using image analysis software (AxioVision 3.1 Carl Zeiss, Germany Kontron Carl Zeiss KS 300, Germany), and the layers were thoroughly examined, using a protocol adapted from Nakashima et al. (15).
Microscopic intensity of lipid deposition in the tunica intima: The formaldehyde-fixed aortic segments were washed with buffer (buffered saline solution) and frozen in liquid nitrogen (–180°C), and the segments were cut using a cryostat at -23°C into 8-µm-thick serial sections (16). The sections were stained with Sudan Red, and the positive areas were visualized at a final magnification of 800X using an image analyzer (Kontron-Zeiss KS-300). Then, the percentage of deposition per analyzed field area was determined (16).
Quantification of collagen: The collagen deposition was quantified in the tunica intima of the aorta using picrosirius-stained sections that were examined under polarized light using a 10X-objective and ImageJ 1.32j software. Collagen deposition was expressed as the percentage of collagen per field area analyzed (17).
Evaluation of mast cells in the aortic layers: In Giemsa-stained sections with a specific color for the mast cells, the mast cells stained in blue were quantified in all aortic layers using a 20X-objective with a final magnification of 500X. Then, the sections were measured to estimate their area in mm2, and the results were expressed as the density of mast cells per mm2, adapted from Kaartinen et al. (18).
A Microsoft Excel® spreadsheet was used for the analysis, and SigmaStat 2.03® software was used for the data analysis. The variable distribution type was verified using the Kolmogorov-Smirnov statistical test. Either Student’s t-test or the Mann-Whitney test was used to compare two groups, and the Kruskal-Wallis test followed by ANOVA (Dunn’s test) and Tukey’s test were used to compare three or more groups. Then, Spearman correlation coefficient was used. Differences in which the probability (p) was less than 5% (p<0.05) were considered statistically significant.
RESULTSDegree of atherosclerosis. The degree of atherosclerosis was significantly higher in the elderly (p≤0.001) (Table 1), mainly when comparing individuals younger than forty years old to those patients who were older than 60 years old (p≤0.001) (Table 2). This increase was also observed in the cases with a cardiovascular cause of death (p = 0.002). There was a positive and significant correlation between the degree of atherosclerosis and age (r = 0.622, p<0.05). Moreover, there was a significant correlation between the degree of atherosclerosis and the presence of cardiovascular disease in all age groups. Such a difference was not observed when the elderly and non-elderly patients did not have cardiovascular disease.
Morphometric analysis of aortas from autopsied patients.
| Groups | DA (cm) | TA (mm2) | TTMA (μm) | TTIA (μm) | LD (%) | CD (%) | DMCA (N/mm2) |
|---|---|---|---|---|---|---|---|
| n = 141 (100%) | Median (Minimum – Maximum) or Median ± SD | ||||||
| Age | |||||||
| Non-elderly, 82 (58.16%) | 1.8 (0.1-11.9) | 27.0 (21.0–30.9) | 1168.8±220.0 | 239.5 (139.6–364.9) | 4.4 (2.4-7.0) | 11.0 (6.9–16.4) | 0 (0–0.4) |
| Elderly, 59 (41.84%) | 7.5 (0.2-11.5) | 31.5 (24.4–39.5) | 1266.5±244.9 | 335.2 (216.3–563.9) | 4.4 (2.4–7.9) | 11.7 (6.0–21.2) | 0 (0–0.3) |
| T = 5710.500; p≤0.001 | T = 4807.000; p = 0.006 | t = 2.415; p = 0.017 | T = 4440.000; p≤0.001 | T = 4232.500; p = 0.857 | T = 4244.000; p = 0.820 | T = 4709.000; p = 0.020 | |
| Gender | |||||||
| Male, 94 (66.66%) | 4.3 (0.1–11.9) | 30.0 (22.0–36.0) | 1196.2±241.9 | 324.7 (191.8–448.5) | 4.4 (2.3–7.0) | 10.3 (6.1–17.0) | 0 (0–0.4) |
| Female, 47 (33.33%) | 3.4 (0.1–11.5) | 28.3 (24.0–33.0) | 1232.3±218.7 | 214.5 (156.2–361.9) | 4.8 (3.0–8.3) | 12.6 (8.3–19.0) | 0 (0–0.1) |
| T = 3149.000; p = 0.412 | T = 3098.500; p = 0.523 | t = 0.838; p = 0.404 | T = 2499.000; p = 0.021 | T = 3485.000; p = 0.519 | T = 3655.500; p = 0.164 | T = 2749.500; p = 0.029 | |
| Ethnicity | |||||||
| Caucasians, 91 (64.54%) | 4.0 (0.1–11.9) | 29.9 (23.0–34.0) | 1216.7±243.1 | 270.5 (173.3–427.7) | 4.9 (2.7–8.1) | 10.2 (5.9–20.6) | 0 (0–0.4) |
| Non-Caucasians, 50 (35.46%) | 4.2 (0.1–11.0) | 28.5 (21.0–33.0) | 1191.9±219.3 | 276.9 (177.9 -402.6) | 3.9 (1.8–6.1) | 11.5 (8.3–16.7) | 0 (0–0.3) |
| T = 3399.500; p = 0.518) | T = 3301.500; p = 0.332 | t = 0.588; p = 0.558 | T = 3245.000; p = 0.932 | T = 3044.000; p = 0.029 | T = 3691.500; p = 0.543 | T = 3240.500; p = 0.216 | |
| Cause of death | |||||||
| Cardiovascular, 62 (43.98%) | 6.4 (0.2 -11.9) | 30.3 (24.5–36.0) | 1235.8±224.7 | 330.9 (207.9–448.1) | 4.1 (2.2–7.0) | 10.9 (6.3–17.0) | 0 (0–0.3) |
| Other, 79 (56.02%) | 3.3 (0.1–11.9) | 27.0 (21.0–32.0) | 1186.3±240.9 | 242.6 (137.6–361.8) | 4.9 (2.7; 7.6) | 11.2 (6.4–20.0) | 0 (0–0.4) |
| T = 5142.000; p = 0.002 | T = 4824.000; p = 0.058 | t = 1.219; p = 0.225 | T = 4618.000; p = 0.007 | T = 4232.000; p = 0.481 | T = 4317.500; p = 0.727 | T = 4352.500; p = 0.940 | |
DA: Degree of atherosclerosis; TA: Thickness of the aorta; TTMA: Thickness of the tunica media of the aorta; TTIA: Thickness of the tunica intima of the aorta; LD: Lipid deposition; CD: Collagen deposition; DMCA: Density of mast cells in the aorta.
Morphometric analysis of aorta from autopsied patients, according to age groups in 10-year intervals.
| AgeGroups | n (%)141(100) | DA (cm) | TA (mm2) | TTMA (μm) | TTIA (μm) | LD (%) | CD (%) | DMCA (N/mm2) |
|---|---|---|---|---|---|---|---|---|
| Median(Minimum – Maximum) or Median ± SD | ||||||||
| 18–27 | 7 (4.96%) | 0.6 (0.1–1.8)1.2.3 | 20.2±5.88 | 1031.6±231.1 | 137.8 (74.8–173.4)4.6 | 6.3 (3.3–9.7) | 12.6 (6.3–21.1) | 0 (0–0) |
| 28 –37 | 19 (13.47%) | 1.1 (0.1–3.6) 1.2.3 | 23.3±5.89 | 1113.2±227.5 | 157.7 (93.3–196.6)5.7 | 3.9 (1.7–5.5) | 11.4 (6.1–21.2) | 0 (0–0.04) |
| 38–47 | 22 (15.60%) | 1.85 (0.1–11)2 | 27.2±7.9 | 1197.5±206.8 | 229.7 (136.1–410.3) | 4.5 (2.4–7.0) | 13.9 (9.6–21.8) | 0 (0–0) |
| 48–57 | 25 (17.74%) | 3.9 (0.4–11.9) | 32.4±9.5 | 1173.9±209.3 | 329.6 (237.3–401.7) | 4.5 (3.1–9.3) | 8.9 (6.7–11.4) | 0 (0–0.08) |
| 58–67 | 36 (25.53%) | 5 (0.2–11.9)1 | 31.2±13.5 | 1248.9±225.6 | 324.0 (269.7–493.5)6.7 | 4.1 (1.7–7.8) | 10.3 (5.5–16.3) | 0 (0–0.02) |
| 68–77 | 25 (17.74%) | 8 (1.8–11.5)2 | 33.1±11.7 | 1287.3±228.8 | 329.3 (200.2–457.4) | 4.0 (2.6–6.7) | 11.7 (6.7–21.1) | 0 (0–0.04) |
| ≥78 | 7 (4.96%) | 9.5 (2.5–10.3)3 | 37.9±13.68.9 | 1348.9±353.4 | 754.3 (335.0–944.4)4.5 | 5.2 (2.7–8.4) | 25.9 (10.6–39.7) | 0 (0–0.08) |
| H = 54.381; p≤0.001 | F = 3.794; p = 0.002 | F = 3.794; p = 0.002 | H = 35.202; p≤0.001 | H = 3.359; p = 0.763 | H = 9.079; p = 0.169 | H = 8.854; p = 0.182 | ||
1,2,3,4,5,6,7 Dunn – p<0.05. 8,9Tukey – p<0.05. DA: Degree of atherosclerosis; TA: Thickness of the aorta; TTMA: Thickness of the tunica media of the aorta; TTIA: Thickness of the tunica intima of the aorta; LD: Lipid deposition; CD: Collagen deposition; DMCA: Density of mast cells in the aorta.
Thickness of the aorta. In general, the aorta was thicker in the elderly patients and in cases with a cardiovascular cause of death. The thickness had a significantly positive correlation with age (r = 0.340, p≤0.001).
Thickness of the tunica intima of the aorta. The tunica intima of the males, elderly males and the cases with a cardiovascular cause of death was significantly thicker (Table 1 and 2). There was a significantly positive correlation between the thickness of the tunica intima and age (r = 0.437, p≤0.001).
Thickness of the tunica media of the aorta. The tunica media was significantly thicker in the elderly. There was no significant difference with respect to gender, ethnicity or cause of death (Table 1 and 2), and there was a significantly positive correlation between the thickness of the tunica media and age (r = 0.268, p = 0.001).
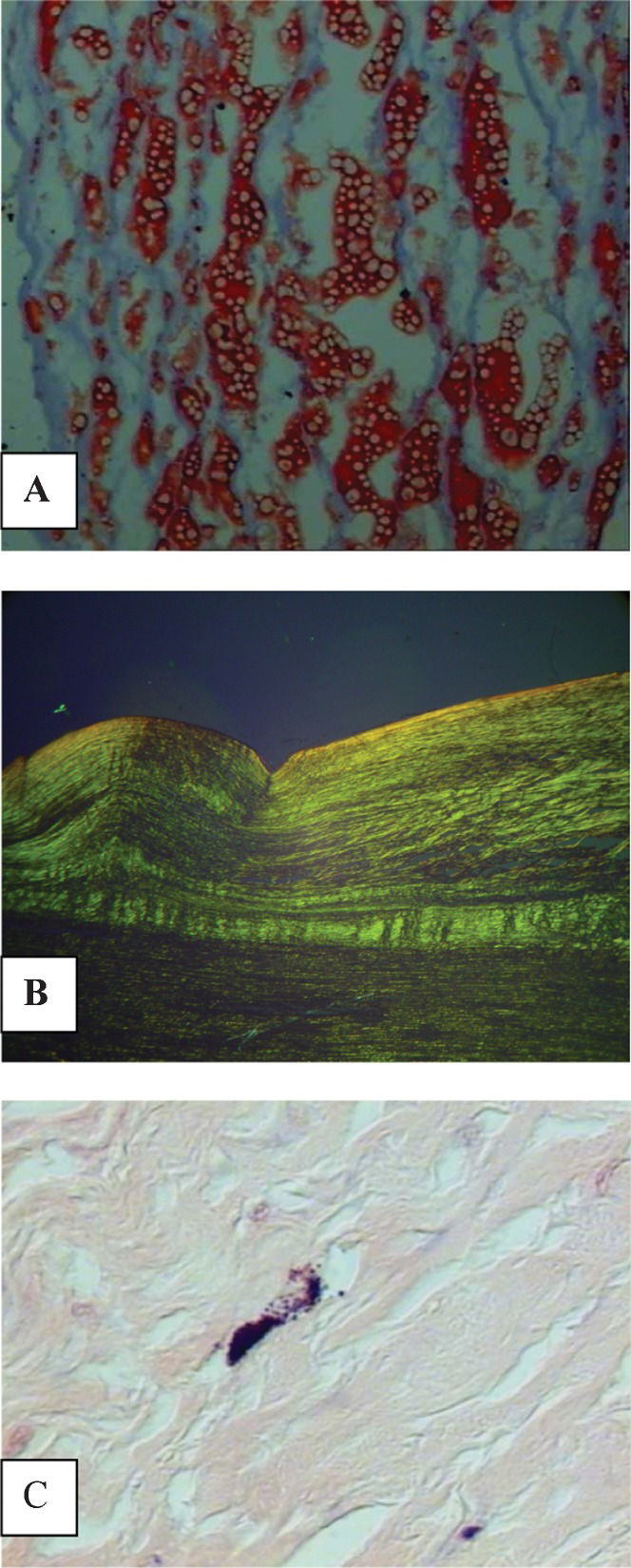
Lipid deposition in the tunica intima. The percentage of lipids in the tunica intima of the aorta was significantly higher in Caucasians (Table 1). In addition, there was no significant difference between this variable and the other variables that were analyzed (Tables 1 and 2), Figure 1A).
(A) Lipid deposition in the tunica intima of the aorta of autopsied patients, identified by red staining (Sudan Red, 800X). (B) Collagen birefringence in the tunica intima of the aorta can be observed under polarized light (Picrosirius, 200X). (C) Purple-stained mast cell in the layers of the aorta (Giemsa, 500X).
Collagen deposition. There was no significant difference between the percentages of collagen and the analyzed variables (Tables 1 and 2, Figure 1B).
Density of mast cells in the aorta. The number of mast cells was significantly higher in males and particularly elderly males (Table 1). There was a significant, positive correlation between age and the density of mast cells (r = 0.214, p = 0.01, Figure 1C).
There was also a significantly positive correlation between the number of mast cells and the degree of atherosclerosis (r = 0.234, p = 0.005).
DISCUSSIONIn this study, the degree of atherosclerosis was more severe starting in the fifth decade of life, which has been well documented in the literature. In a study using the aortas of autopsied patients from 1 to 69 years, atherosclerotic lesions were found to be rare in the first decade of life. Moreover, lesions appeared rapidly in the later decades and were nearly universal after the age of 40 years (19). Our data show that the degree of atherosclerosis usually becomes more severe in the elderly when there are age-associated cardiovascular changes. It is important to emphasize that the differences between the elderly and non-elderly become evident in cases of cardiovascular diseases.
The thickness of the tunica intima had a significant, positive correlation with age and was noticeably thicker in the elderly, males and cases with a cardiovascular cause of death. The same distribution pattern was observed in the tunica media, except for variations associated with gender, ethnicity and cause of death. The outcomes show an increase in the thicknesses of the tunica intima and tunica media with age, and these changes are physiological. Aging causes arterial wall remodeling, which leads to luminal expansion, thickening of the tunica intima and tunica media, increased resistance of the vascular wall, reduction of the regenerative capacity of the endothelium and an increase in the endothelial cell apoptosis rate (20,21). Our data confirm additional findings. Some studies demonstrate that the carotid intima-media thickness may be used as an initial screening test to exclude severe arch atherosclerosis in the general population (22,23). In agreement with our data, an ultrasound study demonstrated that the intimal-medial thickness of the aorta increases with age at a greater rate than the carotid intimal-medial thickness among adolescents and that ultrasonography can be reliably performed in adolescents and young adults to assess the effects of interventions to slow the atherosclerotic process (9). Changes in the tunica intima occur earlier, and other layers of the aorta begin to have such changes in more advanced cases (24). These data were confirmed in this study, which showed more frequent changes in the tunica intima than in the tunica media.
The tunica intima was thicker in male patients, and the tunica media did not show significant changes with respect to gender and ethnicity. These data may be related to the length of time before lesions appear because the tunica intima reacts more quickly to stress factors than the tunica media, as previously mentioned (24). In this study, the increased intimal thickening observed in male patients regardless of age may be associated with the higher prevalence of cardiovascular diseases in males due to, for example, the lack of protective substances such as estrogen hormone that are considered to be anti-inflammatory stabilizers of atheromatous plaques (25).
The percentage of lipids in the tunica intima of the aorta was significantly higher in Caucasian individuals. A study of patients older than 44 years showed a predominance of non-Caucasian patients with an increased prevalence of atherosclerosis (26). The ethnicity data were carefully evaluated in this study, taking the miscegenation of the Brazilian populations into account.
In our cases, the collagen distribution did not exhibit significant variation compared with the analyzed variables, but it was greater in older patients, particularly in those older than 78 years. These data show that collagen deposition as a predictor of the intensity of atherosclerosis is insufficient and should only be considered in cases of older patients, particularly those patients who presented with atherosclerosis for a long time.
There was a significant, positive correlation between age and mast cell density. Mast cells promote atheroma formation by releasing proinflammatory cytokines such as IL-6 and IFN-γ, which regulate vascular cell pro-atherogenic protease expression and local proteolysis in vivo (27). Mast cells may be prematurely recruited, even in young people, with such an increase in mobilization being connected with premature atherosclerotic lesions (1). These data indicate the importance of quantifying these cells as an indicator of atherosclerosis severity because they are involved in both the early and late stages of atherosclerosis. Moreover, these data were confirmed in this study by the positive, significant correlation between the number of mast cells and the degree of atherosclerosis.
Abundant in vitro data have suggested a role for mast cells in both the early and late pathogenesis of atherosclerosis (28). In the early stages of atherogenesis, activated mast cells may increase the amount of low-density lipoprotein in the vascular intima (29). In the late stages, mast cells may secrete proinflammatory cytokines and proteolytic enzymes that make atherosclerotic plaques vulnerable to rupture or erosion (28). Furthermore, a previous study has reported a positive correlation between mast cell number and collagen neoformation (30). The previous information was corroborated by our data, which showed that mast cell number was a good marker for recent lesions. The changes in the number of such cells were more subtle than those changes that occurred with vascular collagen in the cases of severe atherosclerosis.
The mast cell number was increased in male patients. A study demonstrated that the mast cell number could effectively contribute to the inflammatory fibroproliferative processes recognized during the development of atherosclerotic plaques, which is related more with the intensity of atherogenesis than with the individual characteristics (31). According to our data, males, particularly elderly males, presented a greater mast cell number, which was associated with reduced protection against inflammation due to the lack of estrogen (10) and the higher incidence of cardiovascular diseases in males.
Using a semi-quantitative classification in macroscopic analysis is common. Hence, a quantitative analysis was also performed in this study to describe the intensity of atherosclerosis in centimeters, which led to more precise results. The technique used in this study consists of a new macroscopic morphometric method that allowed us to identify various degrees of atherosclerosis rather than the three common grades of mild, moderate and severe. The different methods we used to evaluate aortic atherosclerosis provided us with tools to quantify the degree of atherosclerosis and its relationship with demographic variables through a macroscopic examination. In short, these methods may be a better way to quantify the intensity of such plaques.
Therefore, the present study provides new tools for a more sensitive evaluation of the degree of atherosclerosis in the aorta of autopsied patients. In addition, our results show that macroscopic evaluation is a good predictor of the degree lesion of the atherosclerosis, although a more specific analysis involving the evaluation of the thicknesses of the aortic layers and mast cell count, may better clarify the changes in the components of that vessel.
We would like to thank the following sources of financial support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Fundação de Ensino e Pesquisa de Uberaba (FUNEPU).
No potential conflict of interest was reported.
Ferraz ML, Nascimento DM, and Rorato JP made significant contributions to the conception and data acquisition and were engaged in the design of the present study. Espindula AP, Oliveira LF, Ramalho LS, and Soares MH participated in the sequence alignment and drafted the manuscript. Cavellani CL, Oliveira FA, Pereira SA, Corrêa RR, and Teixeira VP were involved in drafting the manuscript and revising it critically for important intellectual content, providing general supervision for the research group and approving the final version for publication. All authors read and approved the final manuscript.