Fluid volume optimization guided by stroke volume measurements reduces complications of colorectal and high-risk surgeries. We studied whether dehydration or a strong hemodynamic response to general anesthesia increases the probability of fluid responsiveness before surgery begins.
METHODS:Cardiac output, stroke volume, central venous pressure and arterial pressures were measured in 111 patients before general anesthesia (baseline), after induction and stepwise after three bolus infusions of 3 ml/kg of 6% hydroxyethyl starch 130/0.4 (n = 86) or Ringer's lactate (n = 25). A subgroup of 30 patients who received starch were preloaded with 500 ml of Ringer's lactate. Blood volume changes were estimated from the hemoglobin concentration and dehydration was estimated from evidence of renal water conservation in urine samples.
RESULTS:Induction of anesthesia decreased the stroke volume to 62% of baseline (mean); administration of fluids restored this value to 84% (starch) and 68% (Ringer's). The optimized stroke volume index was clustered around 35-40 ml/m2/beat. Additional fluid boluses increased the stroke volume by ≥10% (a sign of fluid responsiveness) in patients with dehydration, as suggested by a low cardiac index and central venous pressure at baseline and by high urinary osmolality, creatinine concentration and specific gravity. Preloading and the hemodynamic response to induction did not correlate with fluid responsiveness. The blood volume expanded 2.3 (starch) and 1.8 (Ringer's) times over the infused volume.
CONCLUSIONS:Fluid volume optimization did not induce a hyperkinetic state but ameliorated the decrease in stroke volume caused by anesthesia. Dehydration, but not the hemodynamic response to the induction, was correlated with fluid responsiveness.
Plasma volume support during surgery and intensive care can be individualized by monitoring the stroke volume (SV) (1,2). The anesthetist then titrates the fluid volume required to place the patient on the top of the Frank-Starling curve of the heart, which is reached when the SV shows only a marginal (or no) increase in response to a higher preload (3). During intensive care, adrenergic drugs are often used as an adjunct to reach this goal; however, during general surgery, bolus infusions of colloid fluids are typically the only tool used to increase preload (4). Due to recent concerns about the safety of colloids, researchers have also attempted to use Ringer's lactate for this purpose (5).
Several reviews have focused on the clinical outcome of patients subjected to this fluid volume optimization (1,2,4). The method appears to have been best evaluated during colorectal surgery. Many studies have also compared technical methods for optimization (6), which is typically applied after anesthesia has been induced. Little is known about why the measurements indicate that 70% of patients are ‘volume responders’ and thus present a functional blood volume deficit even before surgery starts (7).
The present exploratory study assesses the influence of preoperative volume status and the hemodynamic response to the induction of general anesthesia on the probability of fluid responsiveness before surgery begins. The hypotheses were that preoperative dehydration and/or a vigorous hemodynamic reaction would strengthen the indication for this optimization. For this purpose, three successive fluid boluses of hydroxyethyl starch were infused under SV monitoring before surgery began. A secondary aim was to study the efficacy of Ringer's lactate for volume optimization and also for preloading in a subgroup of patients receiving starch.
The preoperative fluid volume status was assessed based on the cardiac index, central venous pressure and signs of renal water conservation in urine samples. SV was the key hemodynamic measure; however, it was usually expressed as stroke volume index (SVI) to facilitate comparisons between patients.
MATERIALS AND METHODSPatientsBetween July 2011 and March 2013, 111 patients (American Society of Anesthesiologists (ASA) class I or II) scheduled for elective laparoscopic or open gastrointestinal surgery performed under intravenous general anesthesia were recruited to participate in the present controlled clinical trial. The reasons for the surgery were suspected or known gastric, colon or rectal cancer. The exclusion criteria were liver or renal dysfunction (liver enzymes>50% of normal or serum creatinine>50% of normal), coagulation disturbances, obstructive pulmonary disease (airway pressure>25 mmHg), atrial fibrillation and mental disorders.
The protocol was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Hangzhou, PR China; No. 2011150, official in charge: Zhangfei Shou). The study was registered at the Chinese Clinical Trial Registry (http://www.chictr.org/en; No. ChiCTR-TNRC-14004479). Written informed consent was obtained from each study subject.
ProcedureThe patients fasted overnight and no premedication was given. When the patient entered the operating theatre, catheterization of the left radial artery and right internal jugular vein was performed under local anesthesia and sedation with midazolam.
All patients received 2-4 l/min of oxygen on an open facemask before general anesthesia was induced (at 8 AM) with intravenous lidocaine, fentanyl and target-controlled infusion of propofol aiming for a plasma concentration of 3-4 µg/ml. Tracheal intubation was facilitated with cisatracurium (0.2 mg/kg). The patients were mechanically ventilated at a tidal volume of 8 ml/kg and a positive end-expiratory pressure of 3 cm H2O. The ventilation rate was 12 breaths/min or adjusted to maintain end-tidal CO2 at 36-44 mmHg. The inspiratory-expiratory ratio was 1:2.
The depth of anesthesia was monitored using a bispectral index (BIS) sensor applied to the forehead. The signal was recorded on a BIS monitor Model A-2000TM (Aspect Medical Systems, Natick, MA) and the level of anesthesia was guided to reach a BIS value between 40 and 60. The drugs used to maintain anesthesia were 1-2% end-tidal sevoflurane, a continuous infusion of propofol (target plasma concentration, 2-3 µg/ml) and/or remifentanil (0.10-0.20 µg/kg/min), with cis-atracurium included intermittently as needed.
The patients' body temperature was maintained at 35.5°C or higher. One patient received i.v. ephedrine (10 mg) when the mean arterial pressure (MAP) fell below 65 mmHg.
Volume optimizationNo fluids were infused during the induction of general anesthesia. Beginning 10 min after tracheal intubation, three bolus infusions of either 6% hydroxyethyl starch 130/0.4 (Voluven®; Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany) (n = 86) or Ringer's lactate (n = 25) were given at a volume of 3 ml/kg over 7 min via an infusion pump (IEC 601–1; Abbott Laboratories, Chicago, IL). The hemodynamic response was recorded 5 min after the end of each bolus infusion. The flat recumbent body position of the patients was maintained and surgery began after all three optimizations had been completed. No other fluids (except drug vehicles) were given during the study period.
A group of 30 patients in the starch group (cases 40 to 69) were preloaded with Ringer's lactate (500 ml) over 2 h, beginning 3 h before the induction of anesthesia. The anesthesia and the research study were managed by anesthetists who were blinded to which subjects received preloading.
MeasurementsThe arterial line was connected to a FloTrac™ sensor from which data were sent to a Vigileo monitor (Software version 3.6; Edwards Lifesciences, Irvine, CA) for analysis. The arterial waveform pulse contour was used to calculate stroke volume index (SVI), cardiac output (CO), cardiac index (CI) and MAP. Monitoring also included central venous pressure (CVP), pulse oximetry, electrocardiography and heart rate; these data were saved digitally on a multifunction monitor (Datex-Ohmeda, Hoevelaken, the Netherlands).
Data on central hemodynamics were collected before and after the induction of anesthesia, just before the first bolus infusion was initiated and then 5 min after each of the three bolus infusions ended. Data not used for calculations were also obtained approximately 30 sec after tracheal intubation. Arterial blood was withdrawn to measure the blood hemoglobin (Hb) concentration, Hb oxygen saturation (SO2) and oxygen tension (pO2) on a GEM Premier 3000 (Instrumentation Lab., Lexington, MA) before anesthesia was induced and while central hemodynamic parameters were measured after each fluid bolus. Duplicate baseline samples ensured a CV for Hb of 1.5%.
Urine was collected via a bladder catheter inserted just after the tracheal intubation had been performed. Samples were sent to a certified clinical chemistry laboratory (Shaoxing People's Hospital Laboratory) for analysis of osmolality (freezing point depression), creatinine concentration (enzymatic method) and specific gravity (refractometry). Urinary changes can mainly be attributed to increased concentrations of metabolic waste due to kidney stimulation to conserve water. Several indices should ideally be assessed (8).
CalculationsThe blood volume (BV) at baseline was estimated using the following anthropometric regression equations (9):
The BV expansion in response to a fluid bolus was calculated based on the change in Hb concentration from before (time 1) and after (time 2) the infusion (9):
The amount of fluid retained in the blood (fluid efficiency) was given by the following equation:
Oxygen delivery (DO2) was obtained from the following:
A bolus was considered to be warranted when raising the SVI by ≥10% (3,4). If a bolus did not fulfill this criterion, the SVI recorded 5 min after the previous bolus infusion ended was utilized as the optimized SVI. If all three bolus infusions raised the SVI by ≥10%, the final value was utilized as the optimized SVI.
Statistical analysisNormally distributed data are presented as the mean (SD) and differences between groups were evaluated using one-way analysis of variance (ANOVA). Data showing a skewed distribution are reported as the median (25th–75th percentile limits) and differences in these data were studied using the Mann-Whitney U test. Incidence data were tested using contingency table analysis. Changes in hemodynamic parameters were expressed as ratios. Relationships between parameters were analyzed using simple linear regression. Receiver operator characteristic (ROC) curves were used to calculate the sensitivity and specificity of selected hemodynamic and urine parameters to predict fluid responsiveness using IBM SPSS Statistics Version 21. Significance was defined as p<0.05.
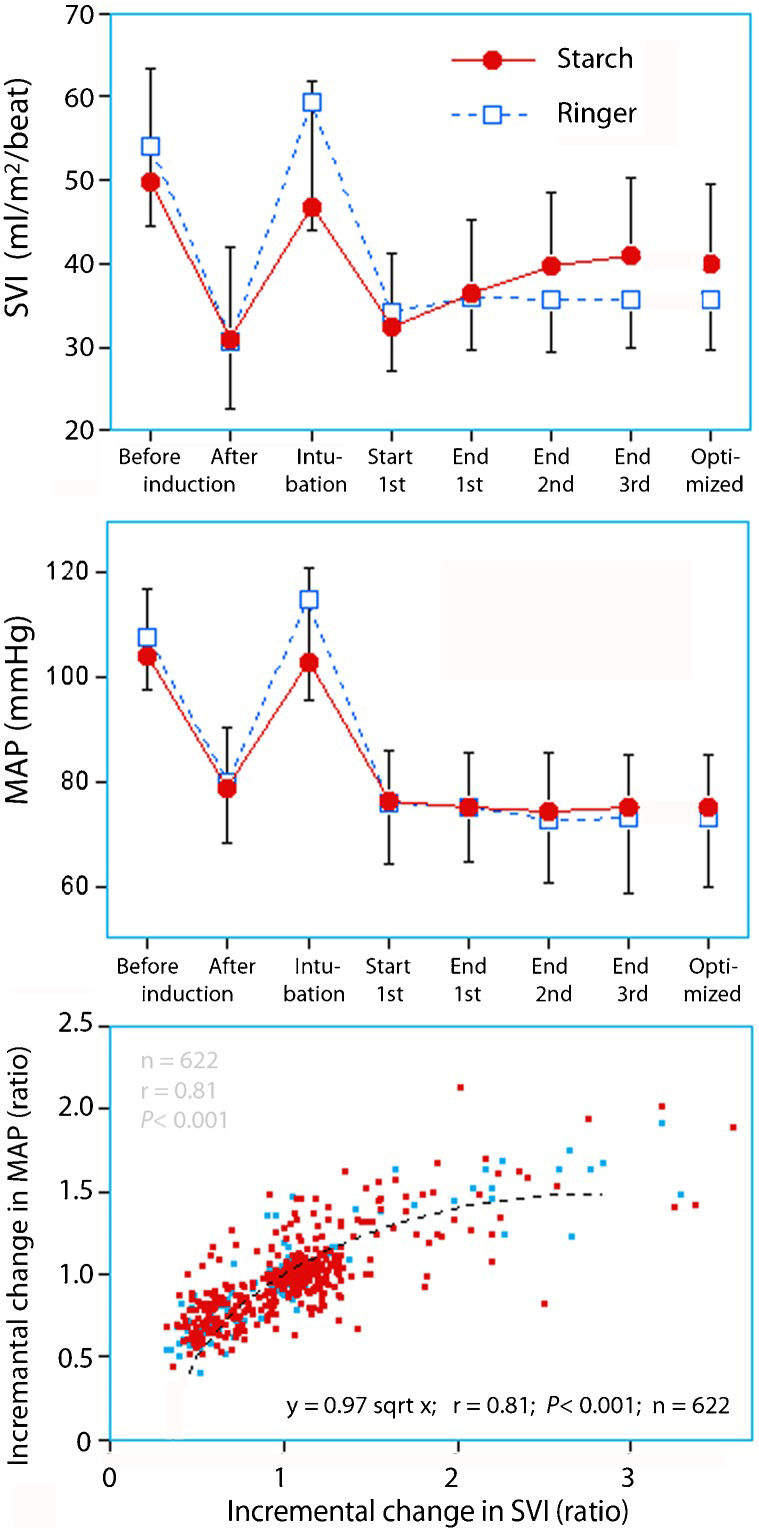
RESULTSHydroxyethyl starchInduction of anesthesia decreased the SVI to 64 (SD, 19)% of baseline. At the end of the three bolus infusions, the SVI was 84 (25)% of baseline. The optimized SVI was also 84 (21)% of baseline (Figure1).
Stroke volume index (SVI, top) and mean arterial pressure (MAP, middle) in patients before and after induction of general anesthesia and after three successive bolus infusions of hydroxyethyl starch or Ringer's lactate. The bottom panel shows the relationship between all incremental changes in SVI and MAP.
A high SVI at baseline was followed by a greater decrease in SVI in response to anesthesia. The relationship was reciprocal and persisted throughout the three bolus infusions (Figure2A–D), which increased SVI by approximately 20% (Figure3A) to reach the optimized SVI of 40 (9) ml/m2/beat (Figure3B).
Preloading did not significantly affect the SVI, CI, MAP or BV expansion; however, two of the three urinary indices of water retention were significantly lower compared to those of patients who did not receive preloading (median U-osmolality of 285 versus 673 mOsmol/kg and U-creatinine 4.9 versus 8.2 mmol/l, both p<0.01).
Among the patients without preloading, those who needed several fluid boluses to become SVI optimized were in a higher state of renal water conservation (higher urinary creatinine concentration, osmolality and specific gravity) compared to those who only required one bolus. These patients also had a lower CI before induction and a lower CVP during the bolus infusion procedure (Table2).
BV expansion exceeded the amount of infused fluid (Table1). After the third bolus, BV had increased by 1247 (426) ml in response to 538 (75) ml of starch. A greater anesthesia-induced drop in MAP was followed by increased fluid efficiency (r = 0.44, p<0.001). The first fluid bolus had an exceptionally high efficiency.
Demographics, hemodynamics and blood volume (BV) expansion. The data are presented according to the contents of the three successive bolus infusions: hydroxyethyl starch or Ringer's lactate.
| Bolus fluid | Starch | Starch + preloading | Ringer's lactate | ANOVA |
|---|---|---|---|---|
| Baseline | ||||
| N | 56 | 30 | 25 | |
| Age (years) | 56 (12) | 58 (13) | 61 (9) | |
| Body weight (kg) | 60 (8) | 59 (9) | 57 (9) | |
| Males (percent) | 29 | 47 | 28 | |
| CI (l/min/m2) | 3.8 (1.0) | 3.8 (0.8) | 3.9 (0.8) | |
| SVI (ml/m2/beat) | 50 (16) | 49 (9) | 54 (9) | |
| MAP (mmHg) | 103 (12) | 105 (14) | 108 (10) | |
| CVP (mmHg) | 4.8 (3.4) | 5.2 (2.2) | 4.9 (2.6) | |
| BV (l) | 4.4 (0.7) | 4.0 (0.8) | 4.1 (0.8) | |
| Bolus infusions | ||||
| SVI before first bolus/baseline (ratio) | 0.67 (0.14) | 0.66 (0.18) | 0.65 (0.15) | |
| SVI after last bolus/baseline (ratio) | 0.86 (0.22) | 0.87 (0.21) | 0.68 (0.15) | p<0.002 |
| MAP before first bolus/baseline (ratio) | 0.73 (0.11) | 0.72 (0.11) | 0.69 (0.11) | |
| MAP after last bolus/baseline (ratio) | 0.74 (0.12) | 0.74 (0.10) | 0.73 (0.14) | |
| Volume of each fluid bolus (ml) | 181 (24) | 176 (26) | 170 (27) | |
| Volume responder (%) | ||||
| 1st bolus | 61 | 70 | 20 | p<0.001 |
| 2nd bolus | 43 | 47 | 13 | p<0.01 |
| 3rd bolus | 23 | 22 | 14 | |
| Volume optimized (%, cumulative) | ||||
| After 1st bolus | 25 | 27 | 74 | p<0.001 |
| After 2nd bolus | 46 | 60 | 81 | p<0.002 |
| After 3rd bolus | 88 | 87 | 89 | |
| Increase in SVI (%) | ||||
| 1st bolus | 15 (15) | 15 (13) | 5 (9) | p<0.01 |
| 2nd bolus | 10 (12) | 11 (16) | 1 (7) | p<0.01 |
| 3rd bolus | 2 (9) | 6 (9) | 3 (10) | |
| BV expansion (ml) | ||||
| 1st bolus | 705 (258) | 622 (247) | 570 (235) | p<0.01 |
| 2nd bolus | 313 (180) | 267 (154) | 188 (131) | p<0.05 |
| 3rd bolus | 300 (304) | 278 (210) | 146 (170) | |
| Fluid efficiency (ratio) | ||||
| 1st bolus | 3.9 (1.3) | 3.5 (1.3) | 3.4 (1.4) | p<0.02 |
| 2nd bolus | 1.8 (1.1) | 1.5 (0.8) | 1.1 (0.8) | |
| 3rd bolus | 1.6 (1.5) | 1.6 (1.1) | 0.9 (1.1) |
The data are presented as the mean (SD) or the incidence expressed as a percentage (%). Incidence data were tested using contingency table analysis.
Induction of anesthesia reduced the SVI to 57 (19)% of baseline. At the end of the three bolus infusions, SVI was at 68 (15)% of baseline (Figure1). The optimized SVI was also 68 (16)% of baseline (lower than starch, p<0.001).
In patients who received starch, a high initial SVI was followed by a greater decrease in response to anesthesia and an optimized SVI that differed from baseline to a greater extent (Figure4A).
The relationship between preoperative and optimized stroke volume index (SVI) in patients receiving Ringer's lactate for fluid volume optimization (top). The fluid efficiency of Ringer's lactate in these patients was greater when there was a profound decrease in mean arterial pressure (MAP, bottom).
Compared with 64% of the patients who received starch, only 20% of the Ringer's patients responded with an increase in SVI of ≥10% in response to the first bolus infusion (p<0.0001). These fluid responders had higher urine-specific gravity than did non-responders (mean, 1.023 versus 1.019; p<0.03).
The CVP at the end of the three infusions was lower after Ringer's than after starch (median, 6 versus 8 mmHg, p<0.01), although this parameter did not differ at baseline (Table1).
BV expansion exceeded the infused volume (Table1). The fluid efficiency for all three boluses was 1.8 (0.6), which should be compared to 2.3 (0.6) for starch (p<0.0001). Greater retention was promoted by a pronounced decrease in the MAP (Figure4B).
Exploratory analysesWarranted boluses: A total of 23% of the “unwarranted” starch infusions (i.e., those that did not increase SVI by ≥10%) were followed by warranted infusions (Table1). Patients in whom several bolus infusions (regardless of order) increased SVI by ≥10% had between 14% and 20% lower SV, SVI, CI and CVP at baseline compared to the others (all differences between p<0.05 and p<0.01). In contrast, the MAP at baseline was virtually identical (mean, 103 mmHg).
Oxygen delivery: DO2 at baseline was 1220 ml/min and decreased both in response to the induction of general anesthesia and following the later infusion of fluid (p<0.001). At the end of the first, second and third bolus infusion, the DO2 values were 671 (210), 635 (203) and 594 (188) ml/min, respectively.
The decrease in DO2 was dependent on fluid responsiveness for those in whom none, 1, 2 or 3 boluses were warranted (i.e., led to an increase in SVI≥10%); the mean decreases in DO2 from the end of the first to the third bolus were 15%, 10%, 7% and 0%, respectively (ANOVA, p<0.02).
Predictive statistics: All three urine indices (urinary creatinine ≥12 mmol/l, specific gravity ≥1.020 and osmolality ≥600 mOsmol/kg) had an area under the ROC curve of 0.65-0.70 when used to predict whether several starch boluses were required to reach the optimized SVI after induction of general anesthesia (preloaded patients excluded). However, the optimal cut-off point for urinary creatinine was ≥10 mmol/l, which showed a sensitivity of 70% and a specificity of 71%. The corresponding sensitivity and specificity values for CI≤3.5 l/min/m2 were 70% and 62%, and for CVP<5 mmHg, they were 50% and 55%, respectively. The areas under these ROC curves were 0.69, 0.65 and 0.61 for urinary creatinine, CI and CVP, respectively (Figure5).
ROC curves for urinary creatinine concentration, urinary osmolality, central venous pressure (CVP) and cardiac index measured before induction of general anesthesia were used to predict whether patients would later have to receive several starch boluses before becoming volume optimized (i.e., at least two boluses increased their stroke volume by ≥10%). The cardiac index is below the reference line, as low values imply a higher likelihood.
Patients who fulfilled the criteria for dehydration based on urinary creatinine, CI and CVP had an 83% likelihood of requiring more than one starch bolus to become SVI optimized (random likelihood, 54%). The likelihood that none or only one bolus would be required was 32%.
When a patient had both urinary creatinine ≥10 mmol/l and CI≤3.5 l/min/m2, the likelihood of requiring more than one starch bolus or only 0–1 starch bolus were 91% and 46%. When neither of these two criteria was fulfilled, the percentages were 68% and 9%.
DISCUSSIONInduction of general anesthesia causes vasodilatation that reduces venous return, causing SVI and CI to decrease. Fluid volume optimization performed in this setting merely served to counteract the drop in SVI that occurred in response to the induction. In the vast majority of patients, the optimized SVI (when fluid could no longer increase SVI) was much lower than the non-optimized SVI measured before induction (Figure1, top). It was simply not possible to create a hyperkinetic hemodynamic state with fluid alone, which might indicate that anesthesia shifted the Frank-Starling curve downward. Even full restoration of baseline SVI was rarely achieved and only occurred in those who had a low SVI at baseline. Overall, two-thirds of the patients who received starch were fluid responders, which agrees with previous findings (7). This proportion is much higher than that observed in conscious preoperative patients (10).
Neither the hemodynamic reaction to anesthesia induction nor the fluid efficiency was able to accurately identify the fluid responder patients (Table2). Instead, the need for more than one fluid bolus before reaching the flat segment of the Frank-Starling curve was associated with discrete signs of dehydration and/or hypovolemia in the form of low CI, SVI and CVP, as well as increased urinary concentrations of metabolic wastes due to renal water conservation.
Urine analysis and hemodynamic responses to three boluses of hydroxyethyl starch. Patients who received preloading with Ringer's lactate are not shown.
| Parameter | 0-1 boluses warranted (N = 26) | 2–3 boluses warranted (N = 30) | Mann-Whitney U test |
|---|---|---|---|
| Urine analysis | |||
| Osmolality (mOsmol/kg) | 536 (439–699) | 687 (618–820) | p<0.04 |
| Creatinine concentration (mmol/l) | 7.2 (4.9–11.8) | 12.3 (6.9–20.1) | p<0.02 |
| Specific gravity | 1.017 (1.015–1.020) | 1.020 (1.019–1.025) | p<0.04 |
| CI and SVI | |||
| CI at baseline (l/min/m2) | 4.2 (3.5–4.7) | 3.3 (2.9–4.1) | p<0.04 |
| CI after three boluses/baseline (ratio) | 0.57 (0.48–0.66) | 0.71 (0.57–0.82) | p<0.02 |
| SVI at baseline (ml/m2/beat) | 51 (44–59) | 42 (38–55) | |
| SVI after induction/baseline (%) | 0.62 (0.55–0.74) | 0.59 (0.50–0.71) | |
| SVI after three boluses/baseline (ratio) | 0.79 (0.71–0.88) | 0.88 (0.80–1.14) | p<0.04 |
| Optimal SVI/baseline (ratio) | 0.72 (0.64–0.85) | 0.92 (0.80–1.13) | p<0.01 |
| MAP | |||
| MAP at baseline (mmHg) | 102 (98–110) | 103 (94–109) | |
| MAP after induction/baseline (ratio) | 0.73 (0.66–0.78) | 0.75 (0.68–0.80) | |
| MAP after three boluses/baseline (ratio) | 0.71 (0.63–0.75) | 0.75 (0.67–0.83) | |
| CVP | |||
| CVP at baseline (mmHg) | 5 (4–6) | 4 (2–6) | |
| CVP after 1st bolus (mmHg) | 7 (6–8) | 5 (4–6) | p<0.001 |
| CVP after 2nd bolus (mmHg) | 8 (7–9) | 6 (5–7) | p<0.001 |
| CVP after 3rd bolus (mmHg) | 9 (8–10) | 7 (6–8) | p<0.001 |
| BV expansion | |||
| BV change after three boluses (l) | 1.4 (1.1–1.6) | 1.2 (1.1–1.5) | |
| Fluid efficiency of three boluses (ratio) | 2.4 (2.1–2.8) | 2.4 (2.0–2.9) |
The data are presented as medians (25th–75th percentile).
The bolus infusions with starch usually caused progressively weaker SVI increases, which is consistent with the Frank-Starling law of the heart; the total increase was 20% for the three bolus infusions in the starch group (Table1, Figure3A). The final effect of the volume optimization was a clustering of the SVI at approximately 35-40 ml/m2/beat, whereas patients with hyperkinetic circulation at baseline tended to achieve somewhat higher values.
Fluid volume optimization with Ringer's lactate was less effective. Here, only one-fifth of the patients appeared to be fluid responders despite the small difference in fluid efficiency between Ringer's lactate and starch. In the clinic, these non-responders would be regarded as being normovolemic, although a comparison with the starch patients suggests that many of them most likely never reached the top of the Frank-Starling curve. This use of insufficient bolus volumes can apparently lead to erroneous conclusions regarding fluid responsiveness.
The same principal relationship between the baseline and optimized SVI was noted for Ringer's lactate and for starch (Figure4A). Animal experiments revealed that starch, but not Ringer's lactate, improved microcirculatory flow and intestinal oxygenation during fluid volume optimization (11). However, Yates et al. recently found that gastrointestinal morbidity after colorectal surgery did not differ between patients who received starch and those who received balanced crystalloid (5). Our data suggest that a fluid volume larger than 3 ml/kg is needed to challenge fluid responsiveness when using a crystalloid in bolus infusions.
Detecting dehydration might be cumbersome in clinical medicine but might be accompanied by slight reductions in CI, SVI and possibly CVP. A serum osmolality ≥300 mOsmol/kg indicates severe (>5%) water depletion (12), whereas urine indices, such as creatinine, osmolality and specific gravity, are capable of revealing dehydration of only 1% (8,13). The urine sample methodology has been developed in sports medicine, where changes in body weight have served as a control.
The cut-off between euhydration and dehydration in healthy adults up to age 70 has been set at 3% of the body weight, which corresponds to levels of urinary creatinine ≥12 mmol/l, specific gravity ≥1.020 and osmolality ≥600 mOsmol/kg (8). Patients whose urine analysis surpassed these limits in our study were more likely to need several boluses before reaching the top of the Frank-Starling curve (Table2, top). Urine analyses were not strong predictors of fluid responsiveness; however, elevated urinary creatinine increased the likelihood (from the random 54% to 70%) that a patient would later require several starch boluses before becoming SVI optimized. This percentage increased to 91% if the high urinary creatinine coexisted with a low CI, which seems to be a strong indication for taking the time required to perform a series of bolus infusions to establish the optimized SVI after inducing general anesthesia. It might be possible to speed up the volume optimization process by infusing colloid fluid until the SVI reaches 40 ml/m2/beat, which is the optimized SVI that most patients achieve. In contrast, the likelihood of encountering a patient who is markedly fluid responsive is low if urinary creatinine is low and CI is on the high side (only 9% of the patients in our study).
The clinical importance of moderately severe dehydration is not well known in perioperative medicine, although a few previous studies suggest a link between dehydration and postoperative complications. Three times as many postoperative complications were observed in hip fracture patients who presented a urine specific gravity ≥1.020 compared with other patients (14). The present study did not record complications, but preloading with 500 ml of Ringer's lactate to prevent dehydration three hours before the study was insufficient to reduce fluid responsiveness and, unfortunately, seemed to invalidate the urine testing. Preloading was not performed in the Ringer's lactate group, as both the preloading and volume optimization with Ringer's lactate were secondary issues in this study.
The pronounced BV expansion from the fluid boluses (fluid efficiency) may be related to hemodynamic depression in response to the induction. Ringer's lactate is known to expand the BV by one-third of the infused volume, but BV expansion is boosted by the arrest of the distribution process that occurs when a patient becomes hypotensive (15). A sudden 20% decrease in the MAP stops the distribution process and the efficiency of crystalloid fluid will be 100% (15). Capillary refill can be expected to further boost the BV expansion in the event that the MAP decreases by nearly 30%, as was observed in the present study. The high fluid efficiency of the first bolus infusion includes the capillary refill that occurs in response to the induction. However, capillary refill in this setting, without a fluid infusion, is modest and dilutes the venous plasma by only 4-7% (16,17).
The fluid efficiency of 6% hydroxyethyl starch 130/0.4 also increased during arterial hypotension, which has not been previously demonstrated. The fluid efficiency is 1.0 in normotensive volunteers (18); however, it apparently increases to 1.5-1.8 during hypotension. As with Ringer's, the fluid efficiency was particularly high during the first bolus infusion.
High fluid efficiency is not only a desired effect but also counteracts the increase in DO2 expected to result from a fluid-induced increase in CO. Induction of general anesthesia reduces DO2 due to its depressive effect on CO; however, the fluid boluses in the present study caused a further reduction by 15% in the non-responders, whereas DO2 was unchanged in the responders. This result indicates that bolus infusions of fluid alone may not be a useful way to increase DO2, as each incremental increase in CO is equal to or smaller than the incremental decrease in Hb. Here, adrenergic drugs would be required to raise DO2 by the 65% needed to reach Shoemaker's original target DO2I of 600 ml/(min m2) (3).
The purpose of using a rigid volume optimization protocol with three straight bolus infusions was to cover the natural course of the SVI optimization process and to limit the bedside influence of the researchers as much as possible. All data were also recorded digitally, which prevented subjective judgement. Certain findings were obtained because this protocol was implemented; for example, 23% of the patients who were already SVI optimized later proved to be fluid responders. This finding is a critical issue for those who use stroke volume monitoring and could be attributed to measurement errors, variability in sympathetic tone or rheological changes caused by the infused fluid.
Another issue is that fluid administration in already SVI-optimized patients reduced DO2. This parameter is greatly influenced by the fluid-induced reciprocal relationship between Hb and CO. The high efficiency of fluid infused after the induction of general anesthesia must be matched by a strong increase in CO (and thereby in SVI) to maintain DO2. When CO does not increase, as in fluid non-responders, a decrease in DO2 occurs. This response must be considered as a risk when infusing fluid without SVI monitoring.
The present study fills a knowledge gap, as virtually all previous studies begin SV monitoring when general anesthesia has already been induced. Limitations include the fact that central hemodynamics were measured using FloTrac/Vigileo, which uses an internal calibration with a higher coefficient of variation compared with that of other arterial waveform pulse contour modes. CO is calculated from arterial pressure, age, gender and a conversion factor based on the skewness and kurtosis of the arterial waveform and on estimates of the vascular compliance and resistance. CO obtained in this way depends less on cardio-pulmonary interactions than on stroke volume variation and pulse pressure variation, which require that patients are mechanically ventilated. The agreement between FloTrac/Vigileo and other methods of measuring CO is considered to be good (19) also during spontaneous breathing (20), although its accuracy and precision in hemodynamically unstable patients remain uncertain.
The value of urine sampling to detect dehydration has been validated against a known deficit in body water in healthy humans but not in those with severe disease. Therefore, patients from ASA class III were not included in this study.
Arterial blood is a better measure of whole-body hemodilution than is peripheral venous blood. Arterial blood shows slightly greater hemodilution than venous blood during infusion of Ringer's lactate, but the arteriovenous difference should dissipate 5 min after the infusion ends (21).
Finally, the patients were studied in blocks of 25-30 and not randomized individually. Patients in the starch group were later randomized to receive either starch or Ringer's lactate during the actual surgery and the results of these experiments will be reported elsewhere.
In conclusion, evidence of dehydration in central hemodynamic parameters and urine samples prior to induction was followed by an increased probability of fluid responsiveness before the commencement of surgery. The SVI averaged 40 ml/m2/beat when the patients were optimized. The bolus infusion volume of Ringer's lactate must be larger than 3 ml/kg to effectively increase the stroke volume.
ACKNOWLEDGMENTSOperation theatre nurse Guofang Meng assisted during the experiments and Qi Liu helped collecting the urine measurements. This project was funded by Qianjiang Talents Project of the Technology Office in Zhejiang province (No. 2012R10033), PR China and by a grant from the Östergötland City Council (No. LiO-297751), Sweden.
AUTHOR CONTRIBUTIONSLi Y organized the study and wrote the appropriate applications. Ying X recruited the patients. Li Y and He R collected the data. Hahn RG was responsible for the conception and design of the study, data analysis and manuscript writing. All authors approved the final version of the manuscript.
No potential conflict of interest was reported.