Hypertonic saline has been proposed to modulate the inflammatory cascade in certain experimental conditions, including pulmonary inflammation caused by inhaled gastric contents. The present study aimed to assess the potential anti-inflammatory effects of administering a single intravenous dose of 7.5% hypertonic saline in an experimental model of acute lung injury induced by hydrochloric acid.
METHODS:Thirty-two pigs were anesthetized and randomly allocated into the following four groups: Sham, which received anesthesia and were observed; HS, which received intravenous 7.5% hypertonic saline solution (4 ml/kg); acute lung injury, which were subjected to acute lung injury with intratracheal hydrochloric acid; and acute lung injury + hypertonic saline, which were subjected to acute lung injury with hydrochloric acid and treated with hypertonic saline. Hemodynamic and ventilatory parameters were recorded over four hours. Subsequently, bronchoalveolar lavage samples were collected at the end of the observation period to measure cytokine levels using an oxidative burst analysis, and lung tissue was collected for a histological analysis.
RESULTS:Hydrochloric acid instillation caused marked changes in respiratory mechanics as well as blood gas and lung parenchyma parameters. Despite the absence of a significant difference between the acute lung injury and acute lung injury + hypertonic saline groups, the acute lung injury animals presented higher neutrophil and tumor necrosis factor alpha (TNF-α), interleukin (IL)-6 and IL-8 levels in the bronchoalveolar lavage analysis. The histopathological analysis revealed pulmonary edema, congestion and alveolar collapse in both groups; however, the differences between groups were not significant. Despite the lower cytokine and neutrophil levels observed in the acute lung injury + hypertonic saline group, significant differences were not observed among the treated and non-treated groups.
CONCLUSIONS:Hypertonic saline infusion after intratracheal hydrochloric acid instillation does not have an effect on inflammatory biomarkers or respiratory gas exchange.
The aspiration of acid gastric contents is the second most common cause of direct acute lung injury (ALI) 1, and it accounts for 10% of all acute respiratory distress syndrome (ARDS) cases 2. Pulmonary inhalation of gastric contents may provoke ALI with different degrees of respiratory compromise and is frequently observed among patients who have depressed airway protective reflexes, such as traumatized patients 3,4. In addition to the potential risk of developing ARDS 5, the inhalation of gastric contents is a common cause of donor lung rejection 6.
Hyperosmolar solutions, such as hypertonic saline, have been tested in an attempt to reduce inflammation in ALI 7–9. However, previous results are contradictory and indicate that the effects of hypertonic saline depend on the type and agent of lung injury and timing of administration 10,11.
The ALI model used in the current study involves the intratracheal instillation of hydrochloric acid (HCl) and attempts to mimic the clinical scenario of gastric content aspiration, wherein a pulmonary lesion is caused by the action of HCl. The acid causes chemical lesions in the lungs, resulting in diffuse injury to the alveoli, release of inflammatory mediators, recruitment of polymorphonuclear cells and promotion of severe edema, and these complications cause a significant reduction in pulmonary compliance and compromise gas exchange 12–14. We hypothesized that hypertonic saline would modulate HCl-induced lung injury. The purpose of this investigation was to test whether a 7.5% hypertonic saline dose could attenuate the inflammatory response evoked by HCl instillation.
METHODSThis study was approved by the Ethics and Animal Investigation Committee of the affiliated institution (CAPPesq 0363/08) and was performed in accordance with the Guide for the Care and Use of Laboratory Animals 15. Thirty-two young female Landrace pigs weighing between 27 and 33 kg (29±2.9) were included in this study. Animals were submitted to 12-hour fasting with free access to water, pre-medicated intramuscularly with ketamine (5 mg/kg) and midazolam (0.5 mg/kg), and catheterized using a marginal ear vein for drug and fluid administration. After sedation, the animals were anesthetized with an intravenous dose of propofol (3-5 mg/kg), orally intubated (6.5 mm internal diameter cuffed endotracheal tube) and placed in the supine position. The lungs were mechanically ventilated (Primus, Dräger, Germany) using pressure-controlled ventilation (FiO2 50%, tidal volume of 8 ml/kg, inspiratory:expiratory (I:E) of 1:2 and positive end-expiratory pressure (PEEP) of 5 cmH2O), with the respiratory frequency adjusted to maintain end-tidal CO2 between 35 and 40 mmHg. To achieve patient-ventilator synchrony, pancuronium bromide (0.3 mg/kg/h) was administered through continuous venous infusion. Anesthesia was maintained with 1.5% isoflurane administered through a calibrated vaporizer (Vapor 2000, Draeger, Lubeck, Germany).
The body temperature was maintained between 37°C and 38°C using a circulating water mattress.
MonitoringAn arterial catheter was inserted into the right femoral artery, and a 7.5 French pulmonary artery catheter that measured continuous cardiac output (Edwards Lifesciences Corp., Irvine, CA) was inserted into the right jugular vein.
The heart rate (HR), mean arterial pressure (MAP), mean pulmonary artery pressure (MPAP), pulmonary artery wedge pressure (PAWP) and central venous pressure (CVP) were obtained directly using a multiparametric monitor (IntelliVue MP40, Phillips, Böblinger, Germany). Using conventional formulae, following indexes were derived: cardiac index (CI), pulmonary vascular resistance index (PVRI) and systemic vascular resistance index (SVRI).
The inspiratory peak pressure, plateau inspiratory pressure, static pulmonary compliance, respiratory frequency and tidal volume were obtained directly from the ventilator monitor. Arterial blood samples were collected at each time point and were immediately analyzed (ABL 555; Radiometer, Copenhagen, Denmark), and the PaO2/FiO2 ratio was calculated.
Study designAfter preparation, all of the animals were submitted to three series of recruitment maneuvers consisting of 30 s of sustained inflation with 20 cmH2O pressure followed by 30 s of regular ventilation. The animals were allowed to stabilize for 30 min and then randomly assigned to four groups: Sham (n=8), hypertonic saline (HS; n=8), acute lung injury (ALI; n=8) and acute lung injury + hypertonic saline (ALI+HS; n=8). ALI was induced in the ALI and ALI+HS groups via intratracheal instillation of HCl through the distal port of a bronchoscope. The ALI+HS animals were treated with 7.5% hypertonic saline (4 mg/kg) 15 min after HCl instillation. The Sham and HS groups served as controls. The animals from the HS group were administered 7.5% hypertonic saline (4 ml/kg) 15 min after the baseline measurement.
The experimental protocol is outlined in Figure 1.
Collecting pointsFollowing a 30-min stabilization period, baseline (BL) measurements were performed. ALI was induced immediately after BL measurements in the ALI and ALI+HS groups. A new series of measurements was collected 30 min after the administration of HCl (T30) and hourly thereafter (T90, T150, T210, T270).
Blood samples were collected at the above time points, and bronchoalveolar lavage (BAL) sampling was performed with 3 × 20 ml phosphate buffered saline (PBS) at T270 through bronchoscopy of the upper right lobe of the lung.
ALI induced by HClA 0.1 N HCl pH 1.0 solution was prepared by the hospital's pharmacy department and instilled at a dose of 4 ml/kg body weight at the level of the carina over a 4 min period through a bronchoscope (Pentax FB-15H, Montvale, NJ). ALI was established when the PaO2/FiO2 ratio fell below 300 mmHg, which was achieved approximately 10 min after hydrochloric acid inhalation.
Neutrophil count and oxidative burst analysisThe BAL samples were pooled, and a 0.2 ml aliquot was transferred to an Eppendorf tube, stained with 0.2% trypan blue solution and counted using a Neubauer chamber.
As previously described 16,17, oxidative burst analysis was performed using a flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA, USA) connected to a computer (Apple, Fremont, CA, USA). BAL cells (2 × 105 cell) were incubated in a 37°C shaking water bath for 30 min with 200 µl of dichloro-dihydro-fluorescein diacetate (DCFH-DA; 0.3 μM, Molecular Probes, Invitrogen, Carlsbad, CA, USA), 200 µl of DCFH-DA and 100 µl of phorbol-12-myristate-13-acetate (PMA; 1 ng/1 µl, Calbiochem, Gibbstown, NJ, USA). In total, 10,000 cellular events were analyzed by Cell Quest Software, and the results were expressed as the geometric mean fluorescence intensity (GMFI).
Cytokine measurementsBAL samples (10 ml) were centrifuged (2,000 rpm, 10 min, 4°C), and the supernatant was stored at -80°C for subsequent analysis. BAL cytokine levels (interleukin (IL)-1, IL-8, IL-10, and tumor necrosis factor alpha (TNF-α)) were assessed using commercially available immunoenzymatic assay (ELISA) kits containing pig-specific monoclonal antibodies, according to the manufacturers' instructions (R&D Systems, Minneapolis, MN, USA). The obtained concentrations were transformed into pg/ml values using a nonlinear regression curve.
Lung histologyAt the end of the study, the trachea was clamped at the end of the inspiratory cycle, and the animal's thorax was opened. Four samples were collected from the middle area of the left apical (West's zone 2) and diaphragmatic lung lobes as well as from the middle of the right apical and diaphragmatic lung lobes. The samples were fixed in a 10% formaldehyde solution for subsequent histologic analysis. The tissue samples were embedded in paraffin, and 5 mm histological sections were stained with hematoxylin and eosin. Two pathologists who were blinded to the study groups performed the histological analyses simultaneously. Examinations included testing for the presence of edema, intra-alveolar and interstitial hemorrhages and polymorphonuclear and mononuclear cell infiltration. Each assessed histological characteristic was attributed a score from 0 to 3 according to the level observed in the tissue (absent (0), mild (1), moderate (2) or severe (3)). The final score for the animal was determined according to the sum of the scores from each lobe (maximum score 12).
Statistical analysisThe results were expressed as the mean±standard deviation (SD). Statistical analyses were performed using a repeated measures analysis of variance (ANOVA) followed by Tukey's test to analyze the group effect on the investigated parameters. A one-way ANOVA was used to analyze the histopathological results. Significance was established at the level of 5% (p<0.05).
RESULTSThe body weights of the animals did not differ between groups. In addition, the administered amount of infused 7.5% hypertonic saline (HS: 171±16 ml; ALI+ HS: 112±11 ml) and HCl (ALI: 119±12 ml; ALI + HS: 112±12 ml) were also similar among the groups.
Hemodynamic and respiratory dataThe hemodynamic measurements were similar for each of the investigated groups throughout the period of observation, and significant changes were not observed in the MAP, CVP, or SVRI values. However, the pulmonary arterial pressure and PVRI values exhibited significant increases after T30 and remained high throughout the observation period in the ALI and ALI+HS groups (Table 1). Following HCl instillation, both lung injury groups exhibited significant reductions in static pulmonary compliance and increases in Pplat (plateau pressure). The PaO2/FiO2 ratio exhibited a significant reduction at T30 in injured animals, or those in the ALI and ALI+HS groups (240±40 and 244±51, respectively, p<0.001), compared with those in the control animals, or the Sham and HS groups (467±37 and 445±57, respectively), and this ratio remained low throughout the observation period (Figure 2).
Hemodynamics and respiratory variables in the control (SHAM and hypertonic saline) and acid lesion (acute lung injury and acute lung injury + hypertonic saline) groups.
| Variable | Group | BL | T30 | T90 | T150 | T210 | T270 |
|---|---|---|---|---|---|---|---|
| CI (L/min/m2) | SHAM | 3.6±0.6 | 3.7±0.5 | 4.0±0.5 | 4.3±0.5 | 4.3±0.7 | 4.3±0.7 |
| HS | 3.7±0.3 | 4.1±0.4 | 4.9±0.4*† | 4.9±0.6* | 4.6±0.7* | 4.5±0.7* | |
| ALI | 3.9±0.6 | 4.2±0.9 | 4.4±0.9 | 4.4±0.8 | 4.2±0.7 | 4.2±0.5 | |
| ALI+HS | 3.5±0.3 | 4.3±0.4* | 4.8±0.5* | 4.8±0.4* | 4.8±0.4* | 4.6±0.5* | |
| HR (bpm) | SHAM | 94±8 | 97±11 | 101±19 | 104±15 | 107±21 | 108±28 |
| HS | 104±15 | 105±11 | 115±15 | 129±18 | 122±18 | 117±10 | |
| ALI | 106±13 | 124±15 | 112±19 | 122±31 | 119±30 | 119±21 | |
| ALI+HS | 92±12 | 114±13 | 120±21 | 121±12 | 124±17 | 121±19 | |
| MAP (mmHg) | SHAM | 54±7 | 59±6* | 69±10* | 66±12* | 61±9* | 61±8* |
| HS | 67±3 | 72±4* | 84±10* | 81±12* | 76±9* | 77±7* | |
| ALI | 70±8 | 80±16* | 80±8* | 84±9* | 82±8* | 81±5* | |
| ALI+HS | 66±3 | 83±17* | 83±11* | 85±11* | 85±12* | 82±8* | |
| MPAP (mmHg) | SHAM | 14±2 | 16±2 | 15±3 | 15±3 | 16±3 | 15±2 |
| HS | 16±2 | 16±1 | 19±1* | 16±6 | 18±2** | 19±2* | |
| ALI | 16±2 | 25±3*†# | 19±3*†# | 22±3*†# | 21±3*†# | 22±4*†# | |
| ALI+HS | 14±3 | 26±5*†# | 21±3*†# | 24±3*†# | 24±4*†# | 23±3*†# | |
| CVP (mmHg) | SHAM | 9±3 | 11±2 | 10±3 | 10±3* | 10±2* | 10±2 |
| HS | 10±2 | 11±1 | 11±1 | 10±1* | 10±2* | 10±2 | |
| ALI | 10±1 | 9±1 | 10±1 | 10±1* | 10±1* | 10±1 | |
| ALI+HS | 9±1 | 11±1 | 9±1 | 10±1* | 10±1* | 10±1 | |
| PVRI (dyne.sec.cm−5.m2) | SHAM | 131±20 | 129±18 | 121±16 | 131±30 | 132±28 | 131±30 |
| HS | 128±15 | 119±30 | 130±40 | 130±27 | 132±31 | 149±28* | |
| ALI | 152±31 | 331±114*†# | 225±51*†# | 266±77*†# | 230±58*†# | 290±51*†# | |
| ALI+HS | 137±35 | 364±127*†# | 207±57*†# | 266±53*†# | 266±66*†# | 263±58*†# | |
| SVRI (dyne.sec.cm−5.m2) | SHAM | 1317±188 | 1249±138 | 1348±158 | 1302±236 | 1362±202 | 1274±111 |
| HS | 1185±124 | 1178±140 | 1157±202 | 1115±200 | 1116±228 | 1172±272 | |
| ALI | 1228±152 | 1343±335 | 1317±354 | 1372±320 | 1419±352 | 1375±187 | |
| ALI+HS | 1266±175 | 1349±291 | 1222±254 | 1247±280 | 1251±274 | 1241±269 | |
| PPlat (cmH2O) | SHAM | 13±1 | 14±1* | 14±1.7* | 14±1.6* | 14±1.6* | 14±1.6* |
| HS | 14±1 | 14±1,8 | 14±1.8* | 15±1.6* | 15±1.9* | 15±2* | |
| ALI | 14±1 | 23±1.3*†# | 21±1.5*†# | 21±1.7*†# | 21±1.8*†# | 21±1.7*†# | |
| ALI+HS | 14±1 | 23±1.6*†# | 21±1.7*†# | 21±1.6*†# | 21±1.2*†# | 21±1.2*†# | |
| Compl (cmH2O) | SHAM | 31±4.1 | 30±4.5 | 29±4 | 29±4.3* | 28±4* | 28±3.6* |
| HS | 29±4.5 | 28±4.6 | 26±3.8* | 26±3.8* | 25±4.4* | 24±4.3* | |
| ALI | 28±4.2 | 14±1.4*†# | 16±2*†# | 16±1.9*†# | 16±1.5*†# | 16±2.5*†# | |
| ALI+HS | 28±4.2 | 13±2*†# | 15±1.6*†# | 15±1.7*†# | 15±1.7*†# | 15±2*†# |
Data are expressed as the mean±standard deviation. CI: cardiac index; HR: heart rate; MAP: mean arterial pressure; MPAP: mean pulmonary arterial pressure; CVP: central venous pressure; PVRI: pulmonary vascular resistance index; SVRI: systemic vascular resistance index; PPlat: plateau pressure; Compl: pulmonary compliance;
*p<0.05 compared with the baseline;
*p<0.01 compared with the Sham group;
# p<0.05 compared with the HS group.
ALI animals showed higher TNF and IL-8 levels relative to the Sham, HS and ALI+HS groups. Higher levels of IL-1B were found in the ALI+HS group, followed by the ALI group (Figure 3).
The ALI and ALI+HS groups (34.4±3.5 and 30.2±7.7 neutrophils × 103/ml, respectively) presented higher BAL neutrophil counts relative to the Sham and HS groups (18.7±1.6 and 17.9±1.5 neutrophils × 103/ml, respectively).
Additionally, the ALI and ALI+HS groups presented significantly higher activity (244.8±130 and 232.6±122 GMFI, respectively) in BAL neutrophils in the PMA-induced burst responses compared with that of the Sham and HS groups (51.7±22 and 57±20.2 GMFI, respectively).
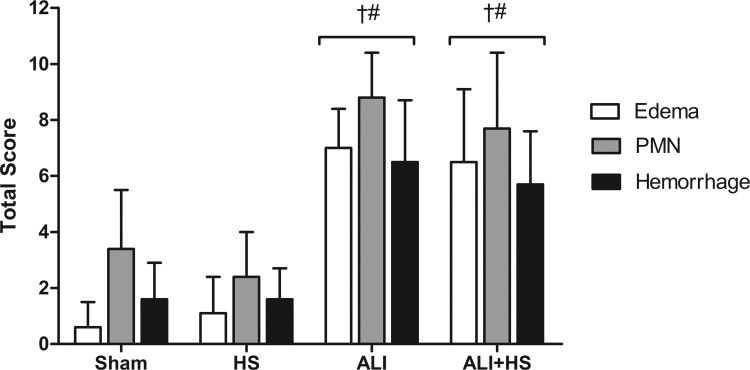
Histological analysisThe score for histological injury was significantly higher in the ALI and ALI+HS groups compared with the Sham and HS groups (Figure 4).
The pattern of lung injury observed in the ALI and ALI+HS groups was heterogeneous and more evident in the diaphragmatic lobes. Examination of these lung tissues revealed large areas of alveolar architecture destruction, hemorrhage, edema and inflammatory polymorphonuclear and mononuclear cell infiltration (Figure 5). However, significant differences were not observed between the scores exhibited by animals in the ALI and ALI+HS groups.
Representative photomicrographs with hematoxylin and eosin (H&E) staining (x200) of the lungs of pigs submitted to acute lung injury. A) Sham group. B) hypertonic saline group. C) acute lung injury group. D) acute lung injury + hypertonic saline group. Extensive alveolar and interstitial inflammatory infiltration was seen in both injury groups.
In the present study, we demonstrated that hypertonic saline infused after intratracheal HCl instillation attenuated increases in BAL neutrophil counts and inflammatory cytokine concentrations. HCl instillation alone induced a severe direct lung injury as evidenced by an intense inflammatory reaction observed in the lung histology, BAL cytokine levels and oxidative burst. Lung function was also adversely affected, which was indicated by decreased gas exchange and reduced lung compliance.
Previous studies have attributed beneficial effects to hypertonic saline in a number of ALI models, such as oleic acid and ischemia/reperfusion-induced lung injury 7,18,19. The use of hypertonic saline solution has also demonstrated potential anti-inflammatory effects related to neutrophil activation 20 in cell cultures as well as in experimental models of sepsis and hemorrhagic shock 21–23. Hypertonic saline solution acts on polymorphonuclear A2 adenosine receptors and causes a feedback mechanism that stimulates cAMP and PKA release, thus blocking neutrophil activation 21–23.
It is believed that hyperosmolar solutions can also decrease pulmonary vascular permeability and leukocyte adhesion molecule expression, especially P-selectin and L-selectin. This expression hinders neutrophil adhesion to the endothelium and may result in reduced lung injury 24,25.
Contrary to the results obtained in studies performed in a HCl-induced lung injury model 8 and an experimental oleic acid-induced lung injury model 7, which demonstrated that pulmonary edema decreased in rats treated with 7.5% hypertonic saline, our histopathological results did not show a significant differences between the ALI and ALI+HS groups with regard to the investigated parameters. However, the ALI+HS group tended to show lower histopathological scores relative to the ALI group, although this difference was not significant. Regarding the control groups, significant differences were not observed between the HS and Sham groups, which demonstrates that the administration of 7.5% hypertonic saline as an isolated agent did not result in worsening lung injury scores.
Alveolar-capillary barrier destruction and increased microvascular permeability are known to trigger the process of lung injury via acid aspiration, which leads to the activation of leukocytes and their migration to pulmonary tissue as well as the production of numerous inflammatory mediators 26. Therefore, our findings of alveolar and interstitial edema and polymorphonuclear and mononuclear cells sequestration in response to injury are consistent with the data reported in the literature.
Although significant differences were not observed between the ALI and ALI+HS groups, higher concentrations of TNF-α and IL-8 in the ALI-group BAL were observed. The concentration of IL-10, an anti-inflammatory cytokine, was significantly lower in the ALI-HS group, which may have been caused by the lower TNF-α values in this group because IL-10 expression is closely related to TNF-α expression 27. However, BAL samples were collected 270 min after acid administration, and the IL-10 concentration may subsequently increase.
The observed increases in MPAP and PVRI suggest that pulmonary hypertension occurred as a result of the initial lung injury triggered by HCl. The two main causes were chemical injury, which led to the destruction of the alveolar-capillary barrier and an increase of pulmonary permeability 28, and pulmonary blood flow redistribution triggered by a physiological process known as hypoxic pulmonary vasoconstriction (HPV), which optimizes the ventilation/perfusion ratio and blood oxygenation by gas exchange.
Regarding the gas exchange, we also found a statistically significant decrease in the PaO2/FiO2 ratio, which was approximately 50% lower in the ALI and ALI+HS groups compared with the other groups. This finding is consistent with the results obtained by Inci et al. 29 in rats after HCl instillation. The PaO2 values, however, were only significantly different among the injury and control groups at the T30 time point and returned to BL values at subsequent time points. Although the PaO2/FiO2 ratio gradually increased throughout the study, these values remained lower in the treated groups compared with the non-injured groups.
Although the PaO2 value and PaO2/FiO2 ratio gradually increased over the observation period, a similar response was not observed in pulmonary compliance, which remained lower for the duration of the study. Vascular occlusion and hypoxic vasoconstriction may shift blood from non-aerated to aerated lung areas, thus contributing to oxygenation improvement.
In addition, to minimize secondary lung injury caused by the ventilator, we limited the pressure-controlled ventilation to a volume of 8 ml/kg and 5 cmH2O PEEP. We also maintained Pplat values below 30 cmH2O throughout the study, which is similar to the standard defined for patients with ARDS [30]. We observed a significant Pplat increase in animals after HCl instillation, which was positively correlated with increases in the mPAP and PVRI values. Moreover, the ALI and ALI+HS groups also showed significantly reduced pulmonary compliance after HCl instillation compared with the control groups. These changes in ventilatory parameters remained throughout the study period and corroborate the results of other studies using HCl- or oleic acid-induced lung injury 29,31.
This ALI model was capable of inducing increased changes in ventilatory parameters, and it also induced the release of inflammatory cytokines in the bronchoalveolar lavage samples in the ALI and ALI+HS groups. The histopathological analysis identified areas of heterogeneous injury characterized by polymorphonuclear infiltrates, alveolar hemorrhage, and edema in the groups subjected to ALI.
Although the use of 7.5% hypertonic saline was shown to be safe in our study, the benefits of its use remain uncertain because we did not observe improvements in any of the investigated parameters among animals in the injury group.
AUTHOR CONTRIBUTIONSHolms CA conducted the study and data analysis. Otsuki DA helped design the study, conduct the study, collect and analyze data and prepare the manuscript. Kahvegian M helped conduct the study. Massoco CO helped conduct the study and data analysis. Fantoni DT designed the study and helped analyze the data. Gutierrez PS performed the histological analysis. Auler Jr JO helped design the study and prepare the manuscript. All of the authors read and approved the final manuscript.
*Presented in part at the 30th International Symposium on Intensive Care and Emergency Medicine, 2010, Brussels.
This work was supported by grants from Fundação de Amparo e Pesquisa do Estado de São Paulo (FAPESP 08/55376-7 and 08/56732-4) and LIM 08 (Medical Investigation Laboratories Institute, Hospital das Clinicas da Faculdade de Medicina).