The aim of this study was to determine the in vitro effect of glutamine and insulin on apoptosis, mitochondrial membrane potential, cell permeability, and inflammatory cytokines in hyperglycemic umbilical vein endothelial cells.
MATERIALS AND METHODS:Human umbilical vein endothelial cells were grown and subjected to glutamine and insulin to examine the effects of these agents on the hyperglycemic state. Mitochondrial function and the production of inflammatory cytokines were assessed using fluorescence analysis and multiple cytotoxicity assays. Apoptosis was analyzed by the terminal deoxynucleotidyl transferase dUTP nick end-labeling assay.
RESULTS:Glutamine maintains the integrity of the mitochondria by reducing the cell permeability and cytochrome c levels and increasing the mitochondrial membrane potential. The cytochrome c level was significantly (p<0.005) reduced when the cells were treated with glutamine. An apoptosis assay revealed significantly reduced apoptosis (p<0.005) in the glutamine-treated cells. Moreover, glutamine alone or in combination with insulin modulated inflammatory cytokine levels. Interleukin-10, interleukin-6, and vascular endothelial growth factor were up-regulated while tumor necrosis factor-α was down-regulated after treatment with glutamine.
CONCLUSIONS:Glutamine, either alone or in combination with insulin, can positively modulate the mitochondrial stress and cell permeability in umbilical vein endothelial cells. Glutamine regulates the expression of inflammatory cytokines and maintains the balance of the mitochondria in a cytoprotective manner.
Hyperglycemia is the abnormality underlying diabetes and several other complications. Chronic hyperglycemia condition initiates a wide range of complications, including cardiovascular disease, which is the most frequent cause of death in the diabetic population 1,2. Hyperglycemia also induces the production of reactive oxygen species (ROS) and, ultimately, DNA damage and apoptosis 3. Apoptosis has been observed in the vascular cells, myocardium, and nerves of diabetic experimental animals and human subjects, although whether it contributes to or is a marker for complications in these tissues is unclear. Vascular diseases are the major cause of morbidity and mortality in diabetic patients. Several studies suggest that the endothelium is the initial site where these vascular complications develop 4. During critical illness, hyperglycemia alone or hyperglycemia coupled with a relative insulin deficiency may directly or indirectly yield a predisposition to a spectrum of complications, such as multi-organ failure and death 5–7. Morphological correlates of these functional abnormalities were not initially identified; however, several later studies showed an increase in apoptosis in several organs affected by diabetes and sepsis, including the eye 8,9, heart, and vascular endothelium 10,11.
Vascular endothelial cells are among the first cells in the body to interact with bacterial endotoxins during sepsis. Sepsis is a very complex and heterogeneous clinical condition that is associated with hyperglycemia and insulin resistance 12. Vascular endothelial cells possess mechanisms that recognize structural patterns of bacterial constituents and initiate the expression of proinflammatory and anti-inflammatory pathways, which are tightly controlled to maintain homeostatic balance 13. However, in severe sepsis, external stimuli, such as a severe infection, can activate the immune cells and unleash a systemic inflammatory response that is expressed through various pathways 14. There is also increasing evidence to suggest that cell death by apoptosis plays an important role in the pathogenesis of severe sepsis/septic shock 15.
Several studies have shown that endothelial cells can undergo apoptosis in response to sepsis-related factors such as Lipopolysaccharide (LPS) and Tumor necrosis factor alpha (TNF-α) 16–18. In addition, studies using in vitro models of infection have demonstrated that certain organisms are capable of inducing endothelial apoptosis 19. Along with the interaction of inflammation with apoptosis in sepsis, mitochondrial dysfunction seems to have a major impact in sepsis patients because it has been closely linked to programmed cell death. Alterations in mitochondrial function have been described in the muscle and liver mitochondria from septic rats and primates. Furthermore, mitochondrial dysfunction has been suggested as a potential mechanism to explain tissue hypoxia despite normal oxygen availability during sepsis 20,21. Pro-inflammatory cytokines, such as interleukin 6 (IL-6), TNF-α, and other molecules, are released during acute inflammation and result in endothelial activation and a significant increase in the expression of endothelial leukocyte adhesion molecule 1, vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and vascular endothelial growth factor (VEGF). These proteins promote leukocyte rolling, adherence, and migration, which initiate inflammation in the endothelium and other cells 22,23. We included IL-10 as an anti-inflammatory marker. Therefore, the aim of this study was to determine the mechanism of endothelial cell apoptosis and the expression of inflammatory cytokines under hyperglycemic conditions and to examine the effects of glutamine and insulin.
MATERIALS AND METHODSCell cultureEndothelial cells were obtained from VEC Technologies (New York, USA). The cells were thawed at 37°C and cultured in T25 flasks coated with 50 µg/ml of fibronectin. The cells were immersed in 5 ml of complete medium (MCDB-B-131), supplemented with 10% FBS, 1% penicillin-streptomycin, and epidermal growth factor (EGF, 10 ng/ml). The cells were incubated at 37 °C with 5% CO2. Trypsin/EDTA (1 ml for each flask) was used to detach the cells upon confluency. All the experiments were performed at passages 2-5.
Cell treatmentThe cells were seeded at 1x104 cells in each well and incubated for 24 hours. Various concentrations of glucose, ranging from a normal value (5 mM) to a hyperglycemic level (20 mM), were added to the individual wells. The hyperglycemic cells (glucose concentration 20 mM) were divided into three groups. In the first group, 40 mM of glutamine was added. In the second group, 1.0 × 10−6 units/ml of insulin was added. In the third group, glutamine (40 mM) and insulin (1.0 × 10−6 units/ml) were added. The cells were then incubated for the required length of time (24 hours). For the cytokine and TUNEL analyses, 0.7×106 cells were grown in T25 flasks using the same treatment groups. The cells were harvested and frozen until required for analysis.
Western blottingThe endothelial cells were first lysed in cold lysis buffer containing 20 mmol/l of TRIS HCl, 140 mmol/l of NaCl, 1 mmol/l of EDTA and complete miniprotease inhibitor cocktail, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, 1 mmol/l NaF, and 1 mmol orthovanadate. The proteins (30 µg) were then loaded on 10% SDS polyacrylamide gels and transferred to activated nitrocellulose membranes. The membranes were blocked with Tris-buffered saline (TBS) containing 5% nonfat milk and incubated overnight with the primary antibodies to IL-10 and TNF-α, obtained from Santa Cruz, at 4°C. Beta-actin was used as a loading control. After extensive washes in TBS, the membranes were incubated for one hour at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibodies, and the proteins were visualized using a chemiluminescence substrate according to the manufacturer's instructions (Amersham Life Sciences).
Multiple cytotoxicity assaysThe Cellomics Multiparameter Cytotoxicity 3–kit was used as previously reported in detail by Cheah et al. 24. The Multiparameter Cytotoxicity 3–kit enables parallel measurements of six independent parameters that monitor cell health, namely, changes in cell permeability, cell loss and nuclear size; changes in mitochondrial membrane potential; cytochrome c release; and morphological changes. Briefly, the cells were plated at 1x104 cells per well for 24 hours. Glucose (5 or 20 mM), glutamine (40 mM), and insulin (1.0 × 10−6 units/ml) were added in different combinations as described in the cell treatment section, and the incubation was continued for 24 hours. The MMP dye and the cell permeability dye were added to the live cells, and the cells were incubated for 1 hour. The cells were fixed, permeabilized, and blocked with 1× blocking buffer before they were incubated with the primary cytochrome c antibody and conjugated secondary antibody for 1 hour. The cells were rinsed three times with wash buffer II, and the plates were analyzed using the Array Scan HCS high content system (Cellomics, PA, USA).
Measurement of transmembrane mitochondrial potentialThe mitochondrial transmembrane potential results from the asymmetric distribution of protons and other ions on the two sides of the inner mitochondrial membrane, which gives rise to the chemical, pH, and electric gradients that are essential for mitochondrial function. The inner side of the inner mitochondrial membrane is negatively charged. Consequently, cationic lipophilic fluorochromes are distributed within the mitochondrial matrix as a function of the Nernst equation describing the transmembrane mitochondrial potential (ψ). Using a cytofluorometer, these dyes were employed to measure variations in the transmembrane potential (ψ) on a per-mitochondrion or per-cell basis.
Terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assayDNA damage was investigated using a 96–well colorimetric apoptosis detection kit (R&D System) according to the manufacturer's instructions. Umbilical vein endothelial cells were cultured and transferred to a 96-well plate (1x105 cells/well). The cells were then fixed with 3.7% buffered formaldehyde for 5 minutes, followed by washing with PBS. The washing was followed by permeabilization of the cells with 100% methanol for 20 minutes and another wash with PBS. Following the manufacturer's protocol, the cells were then subjected to the labeling procedure, and the reaction was stopped with 0.2 N HCl after 30 minutes. The cells were treated with NucleaseTM to generate DNA breaks and to confirm the permeabilization and labeling reactions. An unlabeled control was included to indicate the level of background labeling associated with non-specific binding of the Strep-HRP. The absorbance at 450 nm was measured using a microplate reader.
Cytokine measurementsThe cytokines TNF-α, IL-6, and IL-10 were measured in triplicate using the Protein Bio-Plex Cytokine Assay (Bio-Rad Laboratories). T25 flasks containing 0.7x106 cells were cultured, and the lysate was filter-sterilized (0.22-μm pore size). The protein concentrations were determined, and the Bio-Plex Cytokine Assay (Bio-Rad Laboratories) was conducted according to the manufacturer's protocol. The calculated concentrations for each cytokine were averaged, and the standard deviations were determined. Statistical significance was determined using the t-test, where p<0.05 designated increased/decreased cytokine production in the presence of glutamine or glutamine in combination with insulin.
Statistical analysisEach experiment was performed at least two times. Statistical analysis was performed using one-way analysis of variance (ANOVA).
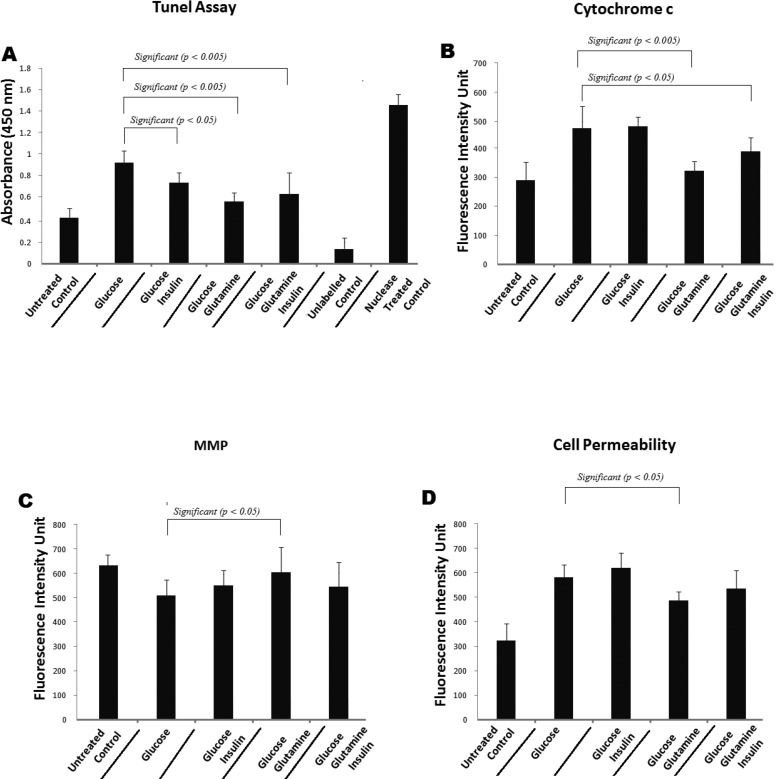
RESULTSGlutamine reduces the cytochrome C levels and apoptosis in hyperglycemic human umbilical vein endothelial cellsUmbilical vein endothelial cells were stained with Hoechst in the presence of glucose (20 mM) alone, 20 mM glucose + 40 mM glutamine, 20 mM glucose + 10−6 M insulin, or 20 mM glucose + 40 mM glutamine + 10−6 M insulin, and the staining intensity was determined. As shown in Figures 1 and 2, glucose alone (20 mM) reduced the number of cells, possibly by apoptosis, as well as reduced the level of cytochrome c. There was a significant change (p<0.005) in the fluorescence intensity in the cells that were treated with glucose along with glutamine. This result indicates that these cells were more viable. In addition, the cytochrome c levels in the endothelial cells treated with glucose (20 mM) + glutamine (40 mM) were significantly (p<0.005) lower than the levels in the cells treated with glucose (20 mM) alone. The cytochrome c levels in the cells treated with glucose (20 mM) + insulin (10−6 M) and glucose (20 mM) + insulin (10−6 M) + glutamine (40 mM) also changed significantly (p<0.05) compared with the levels in the cells treated with glucose (20 mM) alone (Figures 1 and 2B). Similarly, the TUNEL assay revealed a significantly reduced level of apoptosis when the hyperglycemic cells were treated with insulin (p<0.05), glutamine (p<0.005), or glutamine + insulin (p<0.005) (Figure 2A).
Representative images of endothelial cells treated with medium alone (control), glucose alone (20mM), glucose (20mM) + insulin (10−6 M), glucose (20mm) + glutamine (40mM) and glucose (20mM) + glutamine (40mM) + insulin (10−6 M). Cells were stained with Hoechst for nuclear, cell permeability dye, mitochondrial membrane potential dye and cytochrome c. The image from each row was obtained from the same field of the same treatment sample (magnification 20 xs).
Shows (A) TUNEL assay which revealed a significantly reduced apoptosis when hyperglycemic cells were treated with insulin (p<0.05) glutamine (p<0.005) and glutamine + insulin (p<0.005). (B) Cytochrome c intensity for the endothelial cells treated with glucose (20 mM) + glutamine (40 mM) significantly changes when compare to glucose (20 mM) alone. Cytochrome c intensity for the cells treated with glucose (20 mM) + insulin (10−6 M) and glucose (20 mM) + insulin (10−6 M) + glutamine (40 mM) changes insignificantly as compare to glucose (20 mM) alone. (C) and (D) show mitochondrial membrane potential and cell permeability respectively.
According to previous studies, a reduction in the mitochondrial membrane potential occurs when cells undergo oxidative stress and hyperglycemia in the presence of other co-factors. The cells showed a significant change in the MMP when treated with glutamine and a considerable, but not significant, change in the MMP when they were treated with glucose + glutamine + insulin compared with 20 mM glucose only. Glucose (20 mM) + insulin (10−6 M) and glucose (20 mM) + insulin (10−6 M) + glutamine (40 mM) also yielded an increase in the MMP (ΔΨm) compared with the control. These results show that the MMP was reduced after the cells had been treated with 20 mM glucose alone; however, glucose (20 mM) in combination with glutamine (40 mM) was shown to be more protective against the fall in the MMP and oxidative stress-induced cell death (Figure 2C).
Effect of glutamine on cell permeabilityWhen the MMP decline reaches a certain point, the opening of the permeability transition pore (PTP) starts to cause extensive cell damage and, consequently, cell death. The hyperglycemia-induced permeability was reversed when we treated the HUVECs with glucose + glutamine or glucose + glutamine + insulin compared with glucose (20 mM) alone. However, there was a slight increase in the cell permeability when the cells were treated with glucose (20 mM) + insulin (10−6 M) (Figure 2D).
Glutamine and inflammatory cytokinesWhen glucose (20 mM) and insulin (10−6 M) were added to the culture, there was no difference in the expression of IL-6. However, glucose + glutamine significantly increased the expression of IL-6. The combined treatment with glucose (20 mM), glutamine (40 mM), and insulin (10−6 M) also significantly increased the expression of IL-6 (p<0.005). Similarly, treatment with insulin alone did not alter the expression of IL-10, but insulin in combination with glutamine increased the expression of IL-10 in the endothelial cells. A small increase in the expression of VEGF was noted while a reduced expression of TNF-α was observed when the cells were treated with the same concentration of glutamine alone or in combination with insulin (Figure 3A, 3B, 3C, and 3D). Detailed values of all cytokines are given in Table 1. IL-10 is the most important anti-inflammatory cytokine found within the human response. It is a potent inhibitor of Th1 cytokines and a potent activator of monocyte/macrophage proinflammatory cytokine synthesis. In addition, IL-10 attenuates the surface expression of TNF receptors and promotes the shedding of the TNF receptors into the systemic circulation. Furthermore, there is an interesting relationship between IL-10 and the soluble TNF-α receptor. Specifically, when IL-10 increases, it causes an increase in the levels of the soluble TNF-α receptor, which results in decreased TNF-α levels. To confirm this effect, we carried out Western blotting for IL-10 and TNF-α (Figure 4). The levels of both IL-10 and TNF-α changed in the same manner, as shown in Figure 3.
(A) shows that insulin did not alter the expression of IL–10 when treated alone but insulin in combination with glutamine increased IL–10 in endothelial cells (p<0.05). (B) Shows a significantly reduced TNF–α when cells were treated with the same concentration of glutamine in combination with insulin. (C) IL–6 expression which is significantly increased when treated with glutamine in combination with insulin (p<0.005). (D) A mild increase in the expression of VEGF was noted when treated with glutamine or glutamine in combination with insulin.
Cytokine analysis comparing 20 mM glucose, 20 mM glucose + Ins, 20 mM glucose + Gln, and 20 mM glucose + Ins + Gln.
| Variable | Group | n | Mean | p-value |
|---|---|---|---|---|
| Hu IL-6 | 20 mM | 3 | 55.5 | |
| 20 mM+Ins | 3 | 56.2 | NS | |
| 20 mM+Gln | 3 | 73.7 | 0.035 | |
| 20 mM+Ins+Gln | 3 | 91.0 | 0.004 | |
| Hu IL-10 | 20 mM | 3 | 26.3 | |
| 20 mM+Ins | 3 | 27.5 | NS | |
| 20 mM+Gln | 3 | 32.2 | NS | |
| 20 mM+Ins+Gln | 3 | 34.8 | 0.011 | |
| Hu TNF-α | 20 mM | 3 | 13.3 | |
| 20 mM+Ins | 3 | 12.8 | NS | |
| 20 mM+Gln | 3 | 10.7 | NS | |
| 20 mM+Ins+Gln | 3 | 9.7 | 0.006 | |
| Hu VEGF (45) | 20 mM | 3 | 22.0 | |
| 20 mM+Ins | 3 | 20.7 | NS | |
| 20 mM+Gln | 3 | 22.5 | NS | |
| 20 mM+Ins+Gln | 3 | 24.7 | NS |
Our results show that glutamine, either alone or in combination with insulin, disrupts mitochondrial stress and improves cell viability. Anti-inflammatory cytokines were highly expressed in the glutamine-treated cells. With respect to cytochrome C, there was a significant change in the fluorescence intensity in the cytosol in the cells treated with glutamine alone, indicating an increase in the viability of the cells (Figure 2B) on the basis that increased cytochrome c levels in the cells trigger programmed cell death through apoptosis 25,26. Similarly, there was an increase in the mitochondrial potential (Figure 2C).
Our results demonstrate that the cytochrome c levels in cells are increased under hyperglycemic conditions. This protein is known for its function in the mitochondria as a key participant in the life-supporting function of ATP. Our data also support the hypothesis that hyperglycemia, through the production of oxidative stress, could be an apoptotic stimulus that triggers the release of cytochrome c into the cytosol, thereby activating the mitochondrial pathway that leads to the permeabilization of the outer mitochondrial membrane and increasing the level of cytochrome c 27,28. In the cytosol, cytochrome c engages apoptotic protease activating factor-1 (APAF1) and forms the apoptosome. The cell then dies via the apoptotic pathway or necrosis due to the collapse of electron transport, generation of oxygen free radicals, and production of ATP 29,30. As shown in Figure 2B, in our study, the level of cytochrome c in cells cultured in 20 mM glucose (hyperglycemic conditions) was significantly reduced by the addition of glutamine (40 mM), and the viability of the cells was thus increased compared with that of cells incubated with insulin alone or insulin plus glutamine.
Cells induced to undergo apoptosis show an early reduction in the incorporation of ψ-sensitive dyes, which indicates a decrease in the transmembrane potential. This transmembrane potential disruption can be detected in many different cell types, irrespective of the apoptosis-inducing stimulus. The transmembrane potential disruption occurs before the cells exhibit nuclear DNA fragmentation, indicating that the membrane potential change constitutes an early common event of the apoptotic cascade. Purified cells with a low transmembrane potential rapidly proceed to DNA fragmentation. In our study, there was a decrease in the mitochondrial potential under the hyperglycemic condition, suggesting that hyperglycemia could act as an apoptosis-inducing stimulus. The decrease in the mitochondrial potential was reduced in the presence of glutamine or glutamine plus insulin (Figure 2C), although this reduction did not reach significance in the case of glutamine and insulin combined. An intact transmembrane potential (ψ) is indispensable for normal mitochondrial function because cells undergoing apoptosis cease mitochondrial biogenesis at both the translational and transcriptional level 31. Moreover, during apoptosis, mitochondrial inner membrane proteins, including cytochrome c, leak out into the cytosol.
Mitochondria play a central role in the apoptotic process, in which the dissipation of MMP, increased mitochondrial oxidant production, and release of apoptogenic proteins (e.g., apoptosis-inducing factor and cytochrome c) caused by opening of the permeability transition pore are observed. In our study, the cell permeability was increased under hyperglycemic conditions, but the permeability was reduced in the cells treated with glutamine (Figure 2D).
Recent evidence 32 has implicated a general dysregulation of the endothelium, with apoptosis and necrosis as the final pathway of endothelial dysfunction and with mitochondrial dysfunction caused by the central disruption of cellular oxidative function. We therefore hypothesized that mitochondrial dysfunction may also be present in endothelial cells during hyperglycemia and may reflect the degree of systemic injury in patients with severe sepsis and hyperglycemia. Stress-induced hyperglycemia and insulin resistance are exceedingly common in critically ill patients, particularly in those with sepsis. Multiple pathogenic mechanisms are responsible for this metabolic syndrome, with the increased release of proinflammatory mediators and counter-regulatory hormones playing a pivotal role 33. Recent data suggest that hyperglycemia may potentiate the proinflammatory response, while insulin has the opposite effect.
To investigate the possibility that hyperglycemia plays a key role in the development of the inflammatory response in sepsis, we assessed the patterns of IL-6, TNF-α, VEGF, and IL-10 that are associated with severe sepsis. In an exploratory analysis, one study 34 demonstrated that by using multiple cytokine assays, distinct cytokine profiles were found to be associated with the severity of sepsis, the development of organ failure, and death. The inflammatory cytokines IL-1β, IL-6, IL-8, IL-10, and TNF-α have been shown to be associated with the various stages of severe sepsis. To determine whether the same group of cytokines was expressed under hyperglycemic conditions, we assessed the expression of IL-6, TNF-α, VEGF, and IL-10. In our study, we determined the effect of insulin and glutamine on the expression of IL-6, a cytokine with anti–inflammatory and proinflammatory functions. When insulin was added to the cultures, there was no difference in the expression of IL-6 (Figure 3), but glutamine had an additive effect with insulin, and the combination of glutamine and insulin significantly increased the expression of IL-6. Like many other cytokines, IL-6 has both pro–inflammatory and anti-inflammatory properties. Recent evidence generated using IL-6 knockout mice has demonstrated that IL-6, like other members of the gp130 receptor ligand family, acts predominantly as an anti–inflammatory cytokine. IL-6 down-regulates the synthesis of IL-1 and TNF-α and attenuates the synthesis of pro–inflammatory cytokines. Simultaneously, IL-6 inhibits the production of pro–inflammatory cytokines, including GM–CSF, IFNγ, and MIP2. Interestingly, IL-6 may down-regulate TNF-α and IL-β production and may be important in limiting the inflammatory response. Our results demonstrate the same limiting response of IL-6 on TNF-α; however, VEGF was unexpectedly up-regulated by the glutamine treatment. The net results of these immunologic effects place IL-6 among the anti-inflammatory group 35.
The expression of IL-10 (Figure 3) was increased by treatment with glutamine and insulin, with the addition of insulin having an additive effect. IL-10 is the most important anti-inflammatory cytokine in humans. It is a potent inhibitor of the Th1 cytokines and a potent deactivator of monocyte/macrophage pro-inflammatory cytokine synthesis. In addition, IL-10 attenuates the surface expression of TNF receptors and promotes the shedding of the TNF receptors into the systemic circulation 35–37.
The above cytokines have both anti-inflammatory and pro–inflammatory functions 38. Therefore, even during an inflammatory disease such as sepsis, it is important to maintain both the inflammatory and anti-inflammatory cytokines, and this balance seems to be achievable by the effect of glutamine. According to previous reports, glutamine supplementation has been shown to maintain the T–lymphocyte population in the spleen and to significantly enhance the mRNA expression levels of Th1 and Th2 cytokines and TNF-α in the spleens of rats with septic peritonitis 39,40.
Pharmacological supplementation with glutamine helps to maintain the intestinal barrier, modulate cytokine production, and prevent organ injury during sepsis. However, the exact protective mechanism remains to be explored. It has already been demonstrated that glutamine significantly attenuates the plasma levels of cytokines produced by macrophages and endothelial cell necrosis after cecal ligation and puncture in rats 41,42. Recently, it was reported that glutamine treatment directly augmented macrophage TNF-α production in vitro but decreased TNF-α release in vivo, even though the expression of HSP72 was increased in both cases 43. Another report suggests that dietary glutamine administration results in higher inflammatory cytokine production and greater neutrophil recruitment during the early stage of acute lung injury 44.
In conclusion, our data suggest that glutamine alone or in combination with insulin can modulate the production of inflammatory and anti-inflammatory cytokines and maintain the cytokine balance under hyperglycemic conditions, producing a cytoprotective effect. At the same time, our data indicate that glutamine maintains the integrity of the mitochondria, the dysfunction of which in hyperglycemic endothelial cells may reflect the degree of systemic injury in severe sepsis.
AUTHOR CONTRIBUTIONSSafi SZ performed the basic work and wrote the manuscript. Batumalaie K helped with the lab work. Kumar S and Karimian H helped with the reagents. Mansor M, Mohan S, Qvist R, and Yan GOS designed the study and reviewed the manuscript several times. Chinna K and Ahraf MA helped with the statistical analysis.
This work was supported in part by grants (No. RG074/09AFR, and RG528-13HTM (UMRG)) from the University of Malaya. We thank Arokiasamy Vinsent Rayappan (Department of Medicine, Faculty of Medicine, UM) for helping in the cell culture work. We declare there is no conflict of interest.