This study was performed to determine the effect of the tocotrienol-rich fraction on the lifespan and oxidative status of C. elegans under oxidative stress.
METHODLifespan was determined by counting the number of surviving nematodes daily under a dissecting microscope after treatment with hydrogen peroxide and the tocotrienol-rich fraction. The evaluated oxidative markers included lipofuscin, which was measured using a fluorescent microscope, and protein carbonyl and 8-hydroxy-2′-deoxyguanosine, which were measured using commercially available kits.
RESULTSHydrogen peroxide-induced oxidative stress significantly decreased the mean lifespan of C. elegans, which was restored to that of the control by the tocotrienol-rich fraction when administered before or both before and after the hydrogen peroxide. The accumulation of the age marker lipofuscin, which increased with hydrogen peroxide exposure, was decreased with upon treatment with the tocotrienol-rich fraction (p<0.05). The level of 8-hydroxy-2′-deoxyguanosine significantly increased in the hydrogen peroxide-induced group relative to the control. Treatment with the tocotrienol-rich fraction before or after hydrogen peroxide induction also increased the level of 8-hydroxy-2′-deoxyguanosine relative to the control. However, neither hydrogen peroxide nor the tocotrienol-rich fraction treatment affected the protein carbonyl content of the nematodes.
CONCLUSIONThe tocotrienol-rich fraction restored the lifespan of oxidative stress-induced C. elegans and reduced the accumulation of lipofuscin but did not affect protein damage. In addition, DNA oxidation was increased.
Aging occurs when the physiological system experiences a progressive functional decline that results in poor maintenance of homeostasis maintenance and consequently leads to death (1). The free radical theory of aging postulated by Harman states that free radicals that are produced by aerobic metabolism cause the accumulation of oxidative damage and thereby accelerate the aging process (2). Hydrogen peroxide (H2O2) is one of the most abundant reactive oxygen species (ROS) in living cells (3) and has been demonstrated to be involved in the apoptosis pathway, induction of intracellular oxidative stress and acceleration of aging (4,5). H2O2 is toxic and causes further free radical generation, particularly when reacting with reduced transition metals to form hydroxyl radicals (6).
The relationship between the oxidative damage caused by ROS and the aging process has been demonstrated by the discovery of aging biomarkers, such as the autofluorescent pigment lipofuscin, protein carbonyl and 8-hydroxy-2′-deoxyguanosine (8-OHdG), which are often used to measure oxidative damage. Increased levels of these markers are often associated with aging and other age-related diseases (7,8).
In many organisms ranging from invertebrates to humans, increased oxidative damage to lipids, proteins and DNA has been shown to correlate with increasing age (9,10). The nematode C. elegans is commonly used in the study of aging because of its relatively short life cycle, large production of progeny, ease of maintenance in the laboratory and morphological simplicity (11). The aging of C. elegans is characterized by a progressive decline in locomotion, decreased defecation and decreased pharyngeal pumping rate (12–14). In addition, the intestinal cells of C. elegans accumulate an autofluorescent aging pigment called lipofuscin throughout adulthood (14,15). Protein carbonyl has also been reported to accumulate during the aging of C. elegans (16), but changes in the levels of 8-OHdG, which is a DNA damage marker, have not been extensively studied (17–19).
The modulation of endogenous defenses by antioxidant supplementation is regarded as a promising strategy to delay aging. Among the many available antioxidants, vitamin E is considered to be the most established lipid-soluble antioxidant, and it comprises eight isomers: α-, β-, γ- and δ-tocopherol and tocotrienol (20,21). Tocotrienols differ from tocopherol by the presence of an unsaturated isoprenoid tail, which results in a more efficient distribution in the lipid bilayer, and thus they possess more potent antioxidant properties than tocopherol (23,24). Tocotrienols have been shown to reduce protein carbonyl accumulation and subsequently increase the mean lifespan of C. elegans (25). The increase in mean lifespan was attributed to the protective effect of tocotrienols against oxidative stress. However, the role of tocotrienols in the aging process of C. elegans has yet to be fully elucidated.
Because the tocotrienol-rich fraction (TRF) has been found to increase the mean lifespan of C. elegans, this study was performed to further elucidate the effects of TRF on oxidative biomarkers in C. elegans after the induction of oxidative stress.
MATERIALS & METHODSNematode strain and culture conditionsThe wild type C. elegans strain (N2) used in this experiment was obtained from the UKM Molecular Biology Institute (UMBI). All of the maintenance and handling procedures for the nematode were conducted as described previously (26). The nematodes were maintained at 20°C on nematode growth media with E. coli OP50 as a food source (27). To prevent the production of progeny, the nematodes were transferred onto plates containing 40 μM 5-fluoro-2′-deoxyuridine (Sigma-Aldrich, St. Louis, MO, USA) after treatment with H2O2.
The nematodes were divided into six groups and treated accordingly: control, H2O2 induction, TRF treatment, TRF treatment pre-H2O2 induction (TRF+ H2O2), TRF treatment post-H2O2 induction (H2O2+TRF), and TRF treatment pre- and post-H2O2 induction (TRF+ H2O2+TRF). The TRF treatments were given from hatching to day 3 of adulthood to determine the ability of C. elegans to recover from H2O2-induced oxidative stress during the developmental phase.
Nematode growth medium (NGM) with TRF infusionThe TRF was supplied by Sime Darby Bioganic, previously known as Golden Hope Bioganic (Selangor, Malaysia), in its commercial form, TriE, which consists of 15% α-tocopherol, 23% α-tocotrienol, 2% β-tocotrienol, 20% γ-tocotrienol and 11% δ-tocotrienol with 70% vitamin E purity. The optimal dose of TRF for the treatment of C. elegans, 50 μg/ml, was ascertained based on the highest rate of nematode survival after exposure to various concentrations of TRF (Table 1). NGM containing 50 μg/ml TRF was then prepared according to a previous study with minor modifications (25). Briefly, the TRF was dissolved in absolute ethanol containing Tween 80 and then mixed with MilliQ water at a ratio of 1:1, followed by sonication. The TRF solution was then added aseptically to autoclaved nematode growth media before solidification in petri dishes.
Mean lifespan of C. elegans treated with different doses of TRF.
| TRF Concentration | Mean Lifespan |
|---|---|
| Control | 17.79±3.76 |
| (+) control | 18.48±4.09 |
| 50 μg/ml | 20.09±4.02∗),#) |
| 100 μg/ml | 19.79±3.83∗) |
| 200 μg/ml | 18.20±3.94 |
| 400 μg/ml | 18.44±4.30 |
From ANOVA with Tukey's post-hoc test, the mean lifespan of worms treated with 50 and 100 μg/ml TRF was significantly different than that of the control (p<0.05). However, worms treated with 50 μg/ml TRF has the highest mean lifespan, which was significantly different from that of the positive control (Tween 80) (p<0.05);
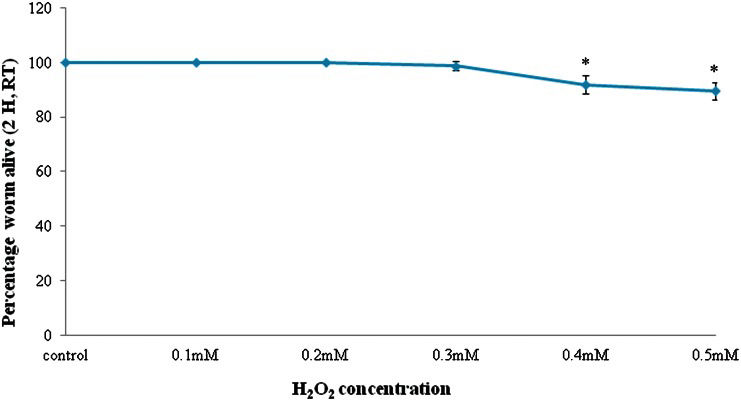
C. elegans nematodes were treated with H2O2 for two hours at the L4 stage (28). The dose of H2O2 used to induce oxidative stress in C. elegans was predetermined to be 0.3 mM because this dose resulted in greater than 90% nematode survival compared with the control. The use of lower doses of H2O2 did not affect nematode survival, and higher doses decreased nematode survival to less than 90% (Figure 1).
Lifespan study of C. elegansThe lifespan study was conducted based on a previously described study (13). Three agar plates containing 50 nematodes each were prepared for each treatment group and assayed simultaneously. The nematodes were counted daily. Nematodes that were recognized as dead, i.e., with a straight body and no response upon probing, were eliminated.
Determination of protein carbonyl contentProtein samples were extracted from C. elegans using the sonication method. The nematodes were sonicated in lysis buffer on ice and then centrifuged for 30 minutes at 4°C. The protein carbonyl content was measured by the OxiSelect™ Protein Carbonyl ELISA Kit (Cell Biolabs, Inc. San Diego, CA) following the manufacturer's protocols. Briefly, BSA standards or protein samples (10 μg/mL) were adsorbed onto a 96-well plate for 2 h at 37°C. The protein carbonyls present in the sample or standard were derivatized with DNP hydrazone and probed with an anti-DNP antibody, followed by an HRP-conjugated secondary antibody. The absorbance was read at 450 nm.
Determination of lipofuscin contentThe presence of lipofuscin was observed using a Zeiss Axiolab fluorescence microscope equipped with an AxioXam MRc camera (Zeiss, Japan). The nematodes from each group were washed from the petri dish and mounted on an agarose pad (2% agarose in M9 buffer containing 0.1% sodium azide). The nematodes were then observed with filter set 09 for blue excitation at 450–490 nm. The fluorescence intensity was measured using KS300 software.
Measurement of 8-OHdGThe C. elegans DNA sample was extracted using the Wizard Genomic DNA Purification kit (Promega, USA) and digested to liberate the individual bases using the 8-OHdG Assay Preparation Reagent set (Wako, Japan). The 8-OHdG level was measured using the 8-OHdG EIA kit (Cayman, USA) following the manufacturer's instructions. The standards and samples were incubated overnight with a tracer and antibody in a plate pre-coated with goat anti-mouse IgG at 4°C. The plate was washed to remove unbound reagent, and the wells were developed using Ellman's Reagent in the dark. The absorbance of the product was read at 405 nm.
Statistical analysisOne-way analysis of variance (ANOVA) and Tukey's post hoc test were used to analyze all of the parameters. p<0.05 was considered statistically significant. The statistical analysis was conducted using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
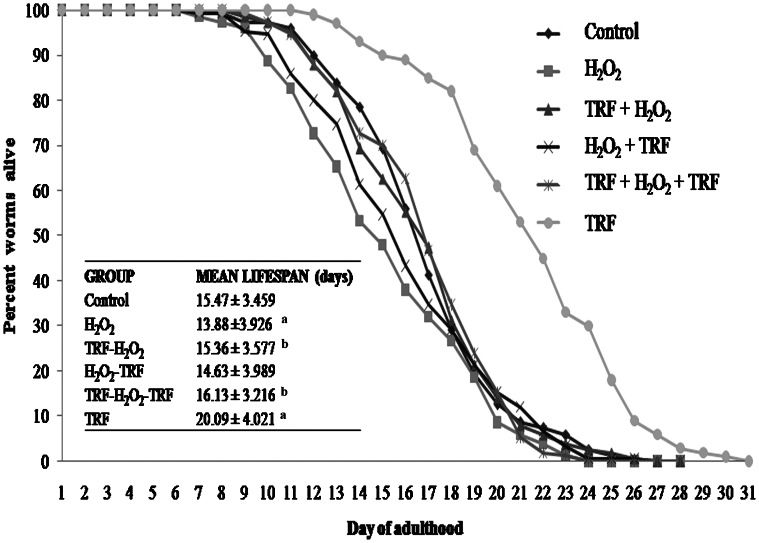
RESULTSLifespan studyH2O2-induced oxidative stress decreased the mean lifespan of C. elegans, whereas the TRF treatment alone increased the mean lifespan (p<0.05) relative to the control (Figure 2). The TRF treatments before and both before and after H2O2 induction also increased the lifespan of the nematodes (p<0.05) relative to nematodes treated with H2O2 alone. However, the mean lifespan of C. elegans was not affected by TRF treatment after H2O2 induction.
Survival curves and mean lifespan of C. elegans treated with TRF. TRF+H2O2+TRF and TRF+H2O2 restored the mean lifespan of the H2O2-treated worms to that of the control group. The results are expressed as the mean ± S.D. with n = 150; asignificantly different (p<0.05) compared with the control; bsignificantly different (p<0.05) compared with H2O2-treated nematodes.
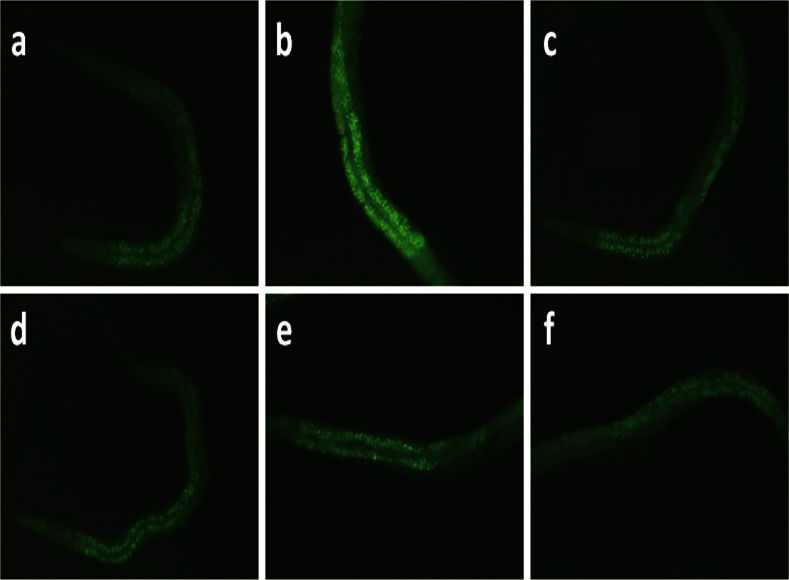
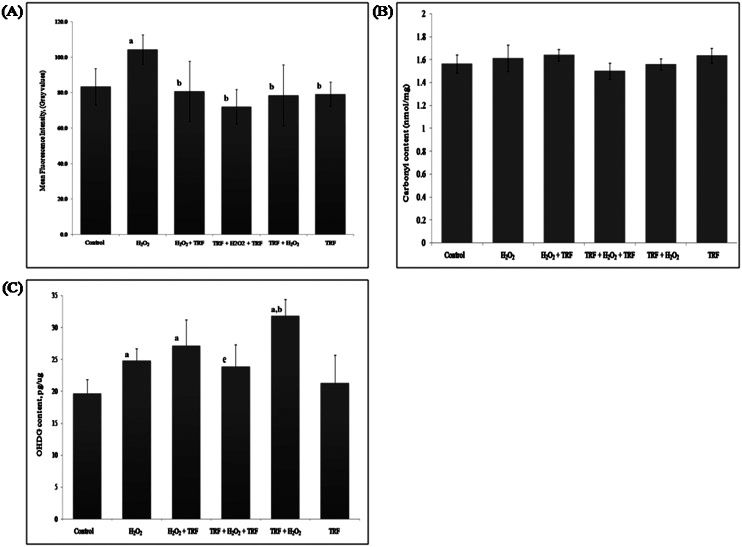
The accumulation of lipofuscin granules in the intestine of a representative nematode from each group is illustrated in Figure 3. Induction of oxidative stress by H2O2 resulted in the accumulation of lipofuscin, which was significantly decreased by all TRF treatments (Figure 4A).
Autofluorescence of lipofuscin granules in the intestines of C. elegans. Fluorescent images were taken using blue excitation light (450-490 nm). Accumulation of lipofuscin granules in the (a) control group, (b) H2O2-treated group, (c) H2O2+TRF group, (d) TRF+H2O2+TRF group, (e) TRF+H2O2 group and (f) TRF group.
Effect of TRF on accumulation of (A) lipofuscin, (B) protein carbonyl and (C) 8-OHdG in C. elegans. The fluorescence intensity of the lipofuscin was measured on day 3 of adulthood. The results are expressed as the mean ± S.D.; asignificantly different (p<0.05) compared with the control; bsignificantly different (p<0.05) compared with the H2O2-treated nematodes; esignificantly different (p<0.05) compared with the TRF + H2O2 group.
H2O2-induced oxidative stress and the TRF treatments had no effect on the protein carbonyl content in C. elegans (Figure 4B).
Effect of TRF treatment on 8-OHdG levelsInduction with H2O2 significantly increased 8-OHdG levels relative to the control (Figure 4C). Treatment with TRF either before or after H2O2 induction also increased the levels of 8-OHdG relative to the control (p<0.05). Continuous treatment with TRF both before and after H2O2 induction effectively decreased the levels of 8-OHdG (p<0.05) relative to application of TRF only prior to the induction of oxidative stress.
DISCUSSIONIn our study, 50 μg/ml TRF was found to be the optimum concentration to increase the mean lifespan of C. elegans. This concentration of TRF is lower than that obtained in previous studies, which used 200 μg/ml vitamin E (mainly tocopherol) or 80 μg/ml TRF to extend the mean lifespan of C. elegans (25). These inconsistencies may be attributed to differences in the tocotrienol isomer composition among studies. Our preliminary results show that induction of oxidative stress by 0.3 mM H2O2 for 2 h was non-lethal, with a survival rate higher than 90% relative to the control. This concentration of H2O2 significantly decreased the sinusoidal body movement of the nematodes as observed under a light microscope.
The mean lifespan of the nematodes was significantly reduced following oxidative stress induced by H2O2, which mediates aging signals (30). The H2O2 treatment may have interfered with the cellular function of C. elegans, thereby leading to a shortened lifespan by increasing intracellular ROS levels (31).
Vitamin E has generally been found to increase the lifespan of C. elegans by slowing development, decreasing fecundity and delaying reproduction (32). Similarly, in the present study, the TRF treatment significantly increased the mean lifespan by 4.6 days (p<0.05). Our finding is similar to that of a previous aging study, which showed that treatment with tocotrienols resulted in a reduction of oxidative stress and an increase in oxidative stress resistance, thereby increasing the mean lifespan of C. elegans (25). A combination of pre- and post-treatments with TRF provided increased protection of the nematodes from oxidative stress relative to pre- or post-treatment alone.
As in other multicellular organisms, the aging of C. elegans is characterized by the accumulation of age-associated autofluorescent lipofuscin in the intestine (12). Lipofuscin is an age pigment that is present in intralysosomal granules and primarily composed of cross-linked protein residues and lipid peroxidation residues formed as a result of iron-catalyzed oxidative processes (33). In this study, lipofuscin accumulation was measured as a marker of aging and as a measure of oxidative damage to lipids in C. elegans. Previously, H2O2 was shown to increase lipofuscin accumulation in an in vitro study (34). In line with this finding, we found in the present study that accumulation of lipofuscin was increased following induction with H2O2. The enhanced formation of lipofuscin granules may be the result of increased cellular free radicals, which accumulate with age (35).
The TRF treatments were able to significantly reduce lipofuscin accumulation in nematodes under oxidative stress. Although the pre-treatment with TRF inhibited the accumulation of lipofuscin, the post-treatment with TRF reflected the function of TRF as not only a chain-breaking antioxidant that prevents the propagation of free radical damage but also a factor that enhances the repair and turnover of damaged macromolecules (25). Carotenoids and α-tocopherol have also been reported to inhibit lipofuscin accumulation by disrupting the chain reactions in lipid peroxidation (36). Taken together, the lifespan and lipofuscin assays demonstrate that oxidative damage plays a role in age-related changes and that TRF treatment promotes resistance to oxidative stress, which leads to an increased mean lifespan for C. elegans.
The protein carbonyl level was measured as an index of protein oxidation. Overall, neither H2O2 induction nor the TRF treatment altered protein carbonyl levels in C. elegans on day 3 of adulthood. Our results were similar to those of a previous study, which found that protein carbonyl levels were unaffected by TRF treatment in young nematodes (25). These findings could be attributed to the pattern of protein carbonyl accumulation, which is time-dependent (37). The carbonyl content was not affected in young nematodes because the steady-state level of carbonyl-bearing proteins increases exponentially only during the last third of the lifespan in animals ranging from C. elegans to man (16).
8-OHdG, which was measured as a marker of DNA damage, is produced by the modification of a guanine base by hydroxyl radicals at the C-8 position (8). The level of 8-OHdG increased when C. elegans nematodes were treated with H2O2, and treatment with TRF further increased the levels of 8-OHdG, which correlated inversely with the mean lifespan of C. elegans. A previous study reported that relatively low levels of oxidative stress promote cellular proliferation rather than causing degeneration or death (38). Because tocotrienol functions as signaling molecule in addition to providing antioxidant activity (39), the observed increase in oxidative DNA damage with TRF treatment may be the result of signal transduction preceding the initiation of cell apoptosis (40) that stimulated cellular regeneration and thus restored the lifespan of C. elegans.
In conclusion, treatment with TRF restored the mean lifespan of C. elegans under oxidative stress and reduced the accumulation of lipofuscin. In addition, a combination of pre- and post-treatments with TRF conferred better protection against oxidative DNA damage than either pre- or post-treatment with TRF alone.
AUTHOR CONTRIBUTIONSGoon JA conceived and designed the study and was responsible for the manuscript review. Zainudin MS and Noralisa AK contributed equally to this manuscript and were involved in the acquisition, analysis and interpretation of the data, and was responsible for the manuscript draft. Wan Zurinah WN provided a critical review of the manuscript.
This work was supported by a grant from the Ministry of Science, Technology and Innovation, Malaysia (Science Fund, 02-01-02-SF 0531) and facilitated by the Department of Biochemistry, Faculty of Medicine, UKM and the UKM Molecular Biology Institute, Kuala Lumpur, Malaysia.
No potential conflict of interest was reported.