To analyze the expression of hypoxia-inducible factors (hypoxia-inducible factor 1A and hypoxia-inducible factor 2A) and aldehyde dehydrogenase proteins in patients with locally advanced breast carcinoma who were subjected to neoadjuvant chemotherapy.
METHODSWe included 90 patients with histologically confirmed stage II and III breast carcinoma who were treated with neoadjuvant chemotherapy between 2000 and 2005. Immunohistochemistry for aldehyde dehydrogenase, hypoxia-inducible factor 1A, and hypoxia-inducible factor 2A was performed before and after neoadjuvant chemotherapy. We analyzed the influence of clinical and pathological features on clinical and pathological response, disease-free survival, and overall survival.
RESULTSAn objective clinical response to neoadjuvant chemotherapy was observed in 80% of patients, with 12% showing a complete pathological response. Among all clinical and pathological parameters, only the expression of hypoxia-inducible factor 1A was associated with a pathological response. A positive association was found between expression of aldehyde dehydrogenase and that of hypoxia-inducible factor 1A before and after chemotherapy. Aldehyde dehydrogenase expression was associated with expression of hypoxia inducible-factor 2A in tumors after neoadjuvant treatment. In a univariate analysis, prognosis was influenced by age, pathological response, metastasis to axillary lymph nodes after neoadjuvant chemotherapy, overexpression of hypoxia-inducible factor 2, and the presence of aldehyde dehydrogenase-positive cells within the primary tumor after neoadjuvant chemotherapy. In a multivariate analysis, only age and the presence of aldehyde dehydrogenase-positive cells after chemotherapy were associated with reduced overall survival.
CONCLUSIONThe presence of aldehyde dehydrogenase-positive cells within the residual tumor after neoadjuvant chemotherapy is associated with an increase in the expression of hypoxia-inducible factor 2A and with poor prognosis in patients with locally advanced breast cancer.
Malignant solid tumors are composed of a heterogeneous cell population. Tumor heterogeneity is considered to be a relevant factor in cancer resistance to therapy and in recurrence (1). The presence of a subpopulation of cells with stem cell properties within the tumor has been widely demonstrated to be responsible for tumor heterogeneity (2–4). In breast carcinomas, the expression or activity of aldehyde dehydrogenase (ALDH) has been reported to be a marker for stem-like cancer cells, based on the observation that cells with ALDH expression have self-renewal and lineage differentiation capacity, as well as the ability to produce tumors in xenograft models. Moreover, the presence of ALDH-positive cells within inflammatory breast carcinomas has been significantly associated with poor survival (5,6).
Although the presence of cancer stem cells could explain tumor heterogeneity and cancer relapse, evidence that tumor cell phenotype is influenced by microenvironmental changes supports the hypothesis that cell plasticity is involved in tumor heterogeneity (7,8). The conversion to a stem-like state can be driven by the epithelial-to-mesenchymal transition (EMT) (9,10). Microenvironmental changes and signaling, such as tissue hypoxia and hypoxia-inducible factor-mediated signaling, can trigger EMT, thereby promoting metastasis and stemness (11,12). It was recently demonstrated that differentiated cells could convert into stem-like cells in normal and neoplastic tissues (7). Additionally, the use of antiangiogenic agents resulted in an increase in cancer-initiating cells in breast cancer xenografts through the generation of tumor hypoxia (13). Hypoxia-inducible factors (HIFs) mediate the processes involved in oxygen homeostasis. HIFs transcriptionally regulate the expression of target genes and represent a link between oxygen sensors and effectors in the cellular adaptation to hypoxia (14). Such observations support the idea that tumor hypoxia and hypoxia signaling are important for cancer heterogeneity, aggressiveness, and dissemination.
Neoadjuvant chemotherapy (NACT) is currently being used as a clinical approach to reduce tumor volume in locally advanced breast cancer and as an alternative method for facilitating the use of breast-conserving therapies (15,16). Although primary chemotherapy has no influence on overall survival, it has been reported that patients who achieve complete pathological response (pCR) have better prognoses than those who do not (17). The results of studies on pathological response and ALDH expression have been controversial (18,19). However, the presence of ALDH-positive cells within a tumor was demonstrated to be a prognostic factor in locally advanced breast cancer patients subjected to primary cytotoxic therapy (18,19). These studies suggest that stem cells could be responsible for tumor resistance and relapse. However, the relationship between the presence of ALDH-positive cells within the primary tumor and the expression of hypoxia-inducible factors (HIFs) has not yet been studied. We analyzed the expression of HIF1A, HIF2A, and ALDH in patients with locally advanced breast carcinoma who were subjected to NACT.
MATERIALS AND METHODSWe prospectively included 90 patients with histologically proven invasive breast carcinoma who were subjected to anthracycline/taxane NACT at our institution between 2000 and 2005. In 2011, we obtained the clinical, pathological, and immunohistochemical (estrogen receptor [ER], progesterone receptor [PgR], and HER2) information from the patients' files and retrieved all paraffin blocks from the primary tumors before and after chemotherapy. A total of 75 blocks before treatment and 67 blocks after treatment provided adequate tumor sampling for immunohistochemical analysis of the expression of ALDH, HIF1A, and HIF2A.
All of the patients were treated with intravenous (iv) epirubicin (50 mg/m2) plus docetaxel (75 mg/m2) on day 1 and then every 21 days in a neoadjuvant setting. The clinical response was evaluated at every visit. The number of NACTs varied from two to seven (median of three cycles) according to the clinical response, on the basis of the primary tumor diameter and the axillary status. The clinical response was defined according to the International Union Against Cancer (UICC) criteria. The patients were classified into two groups: the objective response group, which consisted of all patients with a complete or partial response; and the no-response group, which consisted of all patients with stable or progressive disease. All surgical specimens from the definitive breast procedures were submitted for pathological evaluation and were classified as having complete response (pCR) when no residual invasive carcinoma was observed.
All the patients received adjuvant chemotherapy to complete a total of nine cycles: CMF (cyclophosphamide, 600 mg/m2; methotrexate, 50 mg/m2; fluorouracil, 600 mg/m2 iv on D1 every 21 days) for patients with negative or less than four positive axillary lymph nodes (ALNs), or FEC (fluorouracil, 600 mg/m2; epirubicin, 50 mg/m2; cyclophosphamide, 600 mg/m2 iv on D1 every 21 days) if there were more than three positive lymph nodes. Tamoxifen (20 mg/day) for 60 months was offered to all patients with hormone-positive tumors (ER or PgR). No patients received HER2 target therapy. Radiation therapy was performed in the residual breast parenchyma of all patients who underwent breast-conserving surgery (50 Gy). Patients who underwent mastectomies received adjuvant chest wall radiotherapy if the residual tumors had a diameter of at least 5 cm (at the highest pathological diameter), if ALN metastasis was present, or if skin, muscle, or chest wall invasion was present (50 Gy). The supraclavicular fossa was irradiated if four or more positive ALNs were present.
ImmunohistochemistryAll tissue samples were routinely fixed in 4% neutral formalin and embedded in paraffin. Briefly, 3-μm-thick sections were cut from paraffin blocks containing representative tumor samples. The paraffin sections were de-waxed in xylene, rehydrated through a series of graded alcohols, placed in 10 mM citrate buffer, and subjected to heat retrieval using a vapor lock for 40 min. After heating, the slides were allowed to cool to room temperature and were briefly washed with Tris-buffered saline. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 5 min. Normal serum (Novostain Super ABC kit; Novocastra, Newcastle upon Tyne, UK) was used for 30 min to block nonspecific signals. The following primary antibodies were incubated overnight at room temperature: mouse anti-human HIF1A (1:300, clone H1alpha67; Abcam, Cambridge, MA), mouse anti-human HIF2A (1:800, clone ep190b, Abcam, Cambridge, MA), and mouse anti-human ALDH (1:500, clone 44/ALDH; BD, Franklin Lakes, NJ). Immunohistochemical staining was performed using the Biocare Medical Mach 4 Universal Polymer Detection kit (Biocare, Concord, CA) according to the manufacturer's protocol. The following tissues were used as positive controls: melanoma and tonsil for HIF1A, placenta for HIF2A, and liver for ALDH. Negative controls for immunostaining were prepared by omitting the primary antibody.
Scoring methodsHIF1A was present in the cytoplasm and/or nuclei in a homogeneous pattern in tumor cells. The results were interpreted according to staining intensity and were scored as 0, 1+, 2+, or 3+. Any intensity of staining was considered positive. HIF2A staining occurred mainly in the nucleus, and we quantified HIF2A expression in terms of the percentage (P) and intensity (I) of positive cells. The quick score (QS = P×I) was obtained in samples before and after neoadjuvant therapy. Figures 1 and 2 show the immunohistochemistry for HIF1A and HIF2A in invasive ductal carcinomas. ALDH was present in the cytoplasm. A positive control (mesenchymal cells) was considered for all of the samples analyzed. We considered the cases with evident cytoplasmic staining on at least five tumor cells arranged in a cluster to be positive. Figure 3 shows one negative and some positive patterns for ALDH staining in invasive ductal carcinomas.
HIF1A expression in breast carcinoma. Note that homogeneous staining can be observed in all samples. We classified the samples according to staining intensity from 0 to 3+. Tumors classified as 1+ to 3+ were considered to have positive HIF1A expression. (a) Negative expression (200×); (b) weak positive (1+) expression (200×); moderate positive (2+) expression; (d) strong positive (3+) expression (200×).
HIF2A in breast carcinoma. Note the progressive (0, 1+, 2+, and 3+) expression in invasive carcinoma. The quick score (QS) was calculated as the product of the staining intensity and the percentage of positive cells (QS = I×P). (a) Negative expression (200×); (b) weak positive (1+) expression (200×); (c) moderate positive (2+) expression (200×); (d) strong positive expression (200×).
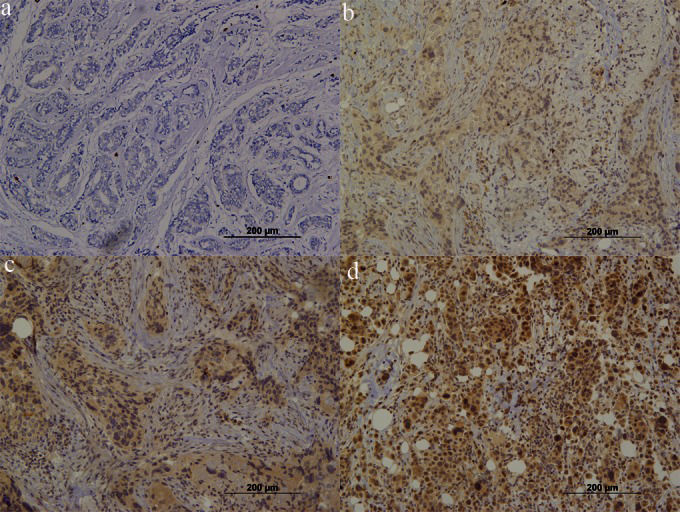
ALDH expression in breast carcinoma. (a) Negative expression. Note the positive expression in mesenchymal cells (internal positive control, 200×). (b) Focal positive expression. Note that some clusters show positive expression in malignant cells (400×). (c) Focal positive cells. Note the variable (weak to strong) brown staining in clusters of malignant cells (200×). (d) Invasive ductal carcinoma with more than 90% of cells showing positive expression of ALDH (400×).
The primary goal was to test the associations between the expression of ALDH, HIF1A, and HIF2A protein and the clinical and pathological response to NACT with the combination of docetaxel and epirubicin. The second objective was to evaluate the impact of the presence of ALDH-positive cells on disease-free and overall survival. We also studied the association between ALDH and HIF expression.
Statistical analysisThe expression of ALDH and HIF protein and other categorical variables were evaluated using a standard x2 test, median test, or Fisher's exact test. Changes in ALDH and HIF1A expression after neoadjuvant treatment were analyzed using McNemar's test. The disease-free survival and overall survival intervals were calculated from the date of diagnosis, and survival curves were derived from Kaplan-Meier estimates and compared using log-rank tests. The influence of prognostic factors on survival was assessed by multivariate analysis (Cox proportional hazard models). JMP, version 7.0.2, SAS, and MedCalc, version 12.1.4, were used for the statistical analyses. The level of significance was established as p<0.05.
EthicsThe ethics committee of the Hospital das Clínicas approved this study (protocol #5764/2000) in accordance with the ethical guidelines of the 1975 Declaration of Helsinki, revised in 1983, and all of the patients signed a consent form.
RESULTSThe patients' mean age was 49±11.5 years; most of them were postmenopausal (55%). According to clinical stage (CS), 11 patients were stage IIa, 33 patients were stage IIb, 15 patients were stage IIIa, and 31 patients were stage IIIb. Invasive ductal carcinoma was diagnosed in 80 (89%) patients, and grade 2 was the most frequent (57.8%) histological grade. The positive rates of ER, PgR, and HER2 were 63.3%, 43.3%, and 27.7%, respectively. An objective clinical response to therapy was observed in 72 patients (80%), and 11 patients (12.2%) achieved pCR. Breast-conserving surgery was possible in 47 patients (52.8%), and 38 patients (42%) were free of ALN metastasis after NACT. The patients' characteristics are summarized in Table 1.
Characteristics of 90 patients with locally advanced breast cancer subjected to neoadjuvant chemotherapy with a combination of epirubicin and docetaxel.
| Age [mean (SD)] | 49 (11.5) |
| Menopausal status [n (%)] | |
| Pre | 40 (44.4%) |
| Post | 50 (55.6%) |
| Clinical stage [n (%)] | |
| II | 44 (48.9%) |
| III | 46 (51.1%) |
| Histology [n (%)] | |
| Ductal | 80 (88.9%) |
| Others | 10 (11.1%) |
| Grade [n (%)] | |
| 1 | 16 (17.8%) |
| 2 | 52 (57.8%) |
| 3 | 22 (24.4%) |
| ER positive [n (%)] | 57 (63.3%) |
| PgR positive [n (%)] | 39 (43.3%) |
| HER2 positive [n (%)] | 25 (27.7%) |
| Objective response [n (%)] | 72 (80%) |
| Breast-conserving surgery rate [n (%)] | 47 (52.8%) |
| pCR [n (%)] | 11 (12.2%) |
| Negative ALN [n (%)] | 38 (42%) |
ER, estrogen receptor; PgR, progesterone receptor; pCR, complete pathological response; ALN, axillary lymph node.
ALDH, HIF1A, and HIF2A protein expression in locally advanced breast carcinomas. ALDH expression was analyzed in 75 and 67 samples before and after treatment, respectively. Positive expression was found in 25 tumors (33%) before treatment and in 32 (47%) tumors after chemotherapy. Paired analysis showed an increase in ALDH protein expression after NACT (McNemar's test, x2 = 3.8; p = 0.04).
Positive expression of HIF1A was observed in 50% and 38% of tumors before and after chemotherapy, respectively (McNemar's test, x2 = 0.4; p = 0.5). The median HIF2A QS was 80 (range 0–270) before NACT and 10 (range 0–270) after NACT. There was a significant reduction in HIF2A expression after NACT (Wilcoxon test, p = 0.0001).
Expression of ALDH, HIF1A, and HIF2A before NACT according to the patients' characteristics. We observed an association between age at diagnosis and the presence of ALDH. In patients with positive ALDH expression, the mean age was 45±9.9 years, compared with 50.8±11.4 years among ALDH-negative patients (p = 0.02). Positive HIF1A expression was found in 76% of grade 3 tumors. Furthermore, positive HIF1A expression was found in 45% and 35% of grade 2 and grade 1 tumors, respectively (p = 0.03).
Expression of ALDH, HIF1A, and HIF2A, and response to NACT. An objective clinical response was observed in 80% of patients. The complete clinical response rate was 23%, and 11 patients (12%) achieved pCR after NACT. The pCR rate was higher in ER-negative than in ER-positive tumors (24% versus 5%, respectively; p = 0.01). No association was observed between PgR or HER2 expression and pCR. We analyzed the accuracy of ALDH, HIF1A, and HIF2A expression as predictive of response on the basis of pCR. ALDH and HIF2A expression did not predict the response. However, the pCR rate in HIF1A-positive patients was 21%, whereas it was 5% in HIF1A-negative patients (p = 0.03). Table 2 lists the results for ALDH and HIF expression as well as the clinical and pathological parameters.
Associations among ALDH, HIF1A, and HIF2A before neoadjuvant chemotherapy, with clinical and pathological parameters, in 75 patients with locally advanced breast cancer subjected to neoadjuvant epirubicin and docetaxel combination.
| ALDH | HIF1A | HIF2A | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Positive(n = 25) | Negative(n = 50) | p∗ | Positive(n = 38) | Negative(n = 37) | p∗ | QS (median) | p∗∗ |
| Age (mean ± SD) | 45±10 | 51±11 | 0.02 | 47±11 | 51±11 | 0.1 | r = −0.11 | 0.3 |
| Menopausal status (n) | ||||||||
| Pre | 18 | 26 | 24 | 19 | 100 | |||
| Post | 7 | 24 | 0.09 | 14 | 18 | 0.3 | 55 | 0.1 |
| Clinical stage (n) | ||||||||
| II | 14 | 23 | 17 | 20 | 80 | |||
| III | 11 | 27 | 0.4 | 21 | 17 | 0.5 | 80 | 0.9 |
| Grade (n) | ||||||||
| 1/2 | 17 | 40 | 25 | 33 | 65 | |||
| 3 | 8 | 10 | 0.2 | 13 | 4 | 0.02 | 100 | 0.2 |
| ER positive (n) | 16 | 32 | 1.0 | 21 | 28 | 0.08 | 80 | 0.8 |
| PgR positive (n) | 11 | 23 | 1.0 | 15 | 20 | 0.2 | 75 | 0.9 |
| HER2 positive (n) | 7 | 13 | 1.0 | 12 | 8 | 0.4 | 60 | 0.4 |
| Clinical response (n) | ||||||||
| OR | 20 | 41 | 33 | 28 | 80 | |||
| NR | 5 | 9 | 1.0 | 5 | 9 | 0.2 | 70 | 0.8 |
| pCR (n) | 4 | 6 | 0.7 | 8 | 2 | 0.03 | 90 | 0.6 |
| ALN (n) | ||||||||
| Positive | 12 | 31 | 18 | 25 | 65 | |||
| Negative | 12 | 19 | 0.4 | 19 | 12 | 0.09 | 100 | 0.08 |
ER, estrogen receptor; OR, objective response; NR, no response; pCR, complete pathological response; ALN, axillary lymph node; ∗ Fisher's exact test; ∗∗median test.
Relationship between ALDH expression and HIF expression. Before NACT, we found positive expression of ALDH in 25 tumors. In this subset of samples, 17 tumors (68%) were positive for HIF1A. HIF1A positivity was observed in 42.8% of ALDH-negative samples. A positive association was observed between ALDH and HIF1A expression (x2 test, p = 0.03). The same expression pattern was observed after chemotherapy. HIF1A positivity was observed in 50% of ALDH-positive tumors and in 21.7% of ALDH-negative tumors (p = 0.03).
The median QS for HIF2A expression in ALDH-positive and -negative tumors was 80 before NACT (range 0–270 and 0–240, respectively; median test, p = 0.9). However, after treatment, the median QS was 55 (range 0–270) in ALDH-positive tumors and 2.5 (range 0–225) in ALDH-negative tumors (median test, p = 0.01).
The presence of ALDH-positive cells after NACT predicted disease-free and overall survival. We further analyzed the influence of clinical and histopathological patterns on disease-free and overall survival. In a univariate analysis, age, axillary status, HER2 protein expression, pathological response, and ALDH protein expression after NACT were identified as significant prognostic factors. Additionally, the presence of ALN metastasis after NACT was more frequent in patients with residual tumors containing ALDH-positive cells (59% versus 41% of ALN metastasis cases, p = 0.04). The worst prognosis was observed among patients with ALDH-positive cells within the residual tumor after NACT and with positive ALNs (overall survival, estimated using a Kaplan-Meier curve, of 24% at 100 months of follow-up). Figure 4 shows the disease-free and overall survival after treatment of patients treated with NACT, according to ALDH protein expression. Multivariate analysis showed that only age (HR = 0.94; CI 95%: 0.91–0.98 for overall survival) and protein expression of ALDH (HR = 2.54; CI 95%: 1.04–6.23 for overall survival) after NACT were significant prognostic factors.
DISCUSSIONBreast cancer is a disease with an adverse prognosis. The presence of stem-like cells or tumor-initiating cells within tumors, defined as ALDH-positive or CD44+/CD24− cells, has been proposed as a relevant factor for treatment resistance as well as disease recurrence and dissemination (4,5,20). In our series, we analyzed the presence of ALDH-positive cells within locally advanced breast cancer, and we observed no relationship between ALDH expression and response to therapy. However, we demonstrated that the presence of ALDH-positive cells within tumors after NACT is an independent prognostic factor. Moreover, the presence of ALDH-positive cells is associated with increased expression of HIF1A and HIF2A, which suggests that hypoxia and HIFs help to maintain stem-like cancer cells within primary tumors.
The existence of cancer stem cells was first demonstrated in acute myeloid leukemia, in which a subset of cells was found that shared some characteristics with normal stem/progenitor cells, such as the expression of stem cell markers, self-renewal ability, and lineage differentiation (2). A similar population of cells was prospectively identified in breast carcinoma (4). According to recent reports, ALDH expression in breast carcinomas can identify a subpopulation of cells enriched with cancer stem cells. Immunohistochemical analyses have shown that ALDH-positive cells are present in 12% to 34% of breast carcinomas (6,18,19). In our study, we observed positive expression in 33% of the tumor samples. Patients with different stages of the disease and different scoring methods may explain the differences among studies.
Although the prospective study of Li et al. demonstrated an increase in the proportion of CD44+/CD24− tumor cells after NACT (21), recent studies have reported controversial data concerning the presence of ALDH-positive cells and response to NACT. Tanei et al. observed significantly lower pCR in ALDH-positive breast tumors (9.5% versus 32% in ALDH-negative tumors) in patients treated with paclitaxel followed by FEC. In contrast, Lee et al. observed a pCR rate of 10% and 41.6% in ALDH-negative and ALDH-positive tumors, respectively, in patients treated with two different regimens of chemotherapy. The pCR rate was only different in patients treated with anthracycline plus taxane (18,19). We did not find any association between ALDH expression and response to therapy. However, we observed that ALDH-positive cells within the residual tumor were associated with an increased risk of developing ALN metastasis after NACT.
The presence of ALN metastasis after NACT is a well-known prognostic factor (17), and a compatible result was obtained in our study. However, we observed that the presence of ALDH-positive cells within the residual primary tumor was a limiting factor for prognosis in ALN-positive patients. We analyzed the overall survival among ALN-positive patients according to the presence of ALDH-positive cells after NACT, and we observed a 62% overall survival rate in ALDH-negative patients, compared with an overall survival rate of 24% in ALDH-positive patients (p = 0.02).
The role of low oxygen tension in the resistance of cancer to therapy has been described for decades (23,24). Tissue oxygen tension less than 20–30 mm Hg has been directly associated with increased resistance to radiation and chemotherapy (25–27). Several biological mechanisms have been described as being relevant to treatment resistance. The direct effect of low oxygen levels and consequent reduced reactive oxygen species (ROS) and free radical production in tumor cells, as well as the expression of genes modulated by the hypoxia-signaling pathway, have been reported to be potential mechanisms (28–30).
Under normoxia, the alpha fractions of HIFs are hydroxylated at two proline-specific residues (Pro-402 and Pro-564), which confers high affinity for von Hippel Lindau (VHL) protein. The HIF-VHL complex is rapidly degraded by the ubiquitin-proteasome machinery. Under low oxygen tension, prolyl-hydroxylase activity is reduced, which consequently decreases HIF-VHL affinity (31,32). HIF alpha protein accumulates, and its dimerization with HIF beta in the nucleus confers a transcriptional function that activates some biological processes, including angiogenesis (through VEGF), inhibition of apoptosis, a glucose metabolism shift favoring glycolysis, and invasion/metastasis (33).
Docetaxel is a cytotoxic drug from the taxane class that is a semisynthetic analog of paclitaxel, which is extracted from the bark of the rare Pacific yew tree Taxus brevifolia (24). Docetaxel has a potent antimitotic action, as it binds to and stabilizes microtubules during metaphase and anaphase (34,35), and in combination with anthracycline, it comprises one of the most potent chemotherapy regimens for breast cancer. Combined docetaxel and anthracycline therapy can reduce blood flow and may account for HIF modulation (23,25). Suppression of HIF2A has been demonstrated to lead to differentiation of neuroblastoma and glioma stem/initiating cells (12,36,37). In breast cancer, HIFs are key mediators of angiogenesis and metastasis (33). Tumor hypoxia can facilitate intravasation, and normalization of the vasculature can reduce metastasis (38). Anti-VEGF therapy increases tumor invasiveness and metastasis, and a recent report demonstrated that anti-VEGF therapy increased the cancer stem cell population through hypoxia pathway signaling in xenograft models (13,39,40). Additionally, clinical studies have demonstrated only limited benefits of antiangiogenic therapy for breast cancer (41).
We observed an overall reduction in HIF1A and HIF2A expression after NACT using a combination of a taxane and anthracycline. However, ALDH protein expression was increased after treatment, and the presence of ALDH-positive cells within the residual tumor was the most important prognostic factor in patients subjected to NACT. Interestingly, HIF2A expression did not decrease in tumors with residual ALDH+ cells after NACT. The role of HIFs in controlling the stem-like cancer cell population has become evident in recent publications. Louie et al. demonstrated that the stem-like breast cancer cell subpopulation could be expanded through repetitive hypoxia/reoxygenation cycles without genetic manipulation (42). These data support previous experimental findings that HIF signaling can control the “stemness” phenotype (12,37). Therefore, the use of HIF-targeted therapy may be effective in reducing or eradicating stem-like cells, thereby improving overall survival.
The presence of ALDH-positive cells within residual tumors after NACT is associated with increased HIF2A expression and poor prognoses in patients with locally advanced breast cancer. An association was found between ALDH expression in tumor cells and HIF1A expression in locally advanced breast carcinomas.
AUTHOR CONTRIBUTIONSTiezzi DG participated in the design of the study and the recruitment and treatment of patients, drafted the manuscript and performed the statistical analysis. Andrade JM participated in the design and coordination of the study and helped to draft the manuscript. Tiezzi MG performed the immunohistochemical analysis. Marana HR participated in the design of the study and in the recruitment and treatment of patients. Mandarano LR, Sousa CB and Clagnan WS helped with the data collection and paraffin block selection.
The authors thank Ms. Ana Maria Anselmi Dorigan for her excellent technical assistance. This research was supported by a grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (process 2008/09294-9) and by Fundação de Amparo ao Ensino, Pesquisa e Assistência (FAEPA) do HCFMRP – USP, Brazil.
No potential conflict of interest was reported.