To evaluate the effectiveness and safety of pleurodesis carried out entirely on an outpatient basis in patients with recurrent malignant pleural effusions and Karnofsky Performance Status scores ≤70.
METHODSThis study was a prospective trial comprising patients with symptomatic recurrent malignant pleural effusion and Karnofsky Performance Status scores ≤70 but >30. All selected patients underwent pleural catheter placement (14 Fr) in an outpatient facility. When chest radiography revealed post-drainage lung expansion of >90%, pleurodesis (3 g of talc) was performed. Catheters were maintained until the daily output was <100 mL/day. The patients were evaluated in the first month and every three months thereafter for fluid recurrence, the need for additional procedures, and complications.
RESULTSDuring the study period (January 2005 to July 2007), 64 patients (24 men, 40 women), with an average age of 61.4 years, underwent elective chest tube drainage. Primary sites of the underlying malignancy were breast (27), lung (22), and others (15). Sixty-six pleural catheters were placed (bilaterally in 2 patients), and 52 talc pleurodesis procedures were performed. Fourteen patients had a trapped lung and were excluded from the trial. No complications were observed during catheter placement or pleurodesis. Post-pleurodesis complications included catheter obstruction (4 patients) and empyema (1). The average drainage time was 9.9 days. The recurrence rate observed in patients that were alive 30 days after pleurodesis was 13.9% (5/36 patients). Six patients required additional procedures after the pleurodesis. The average survival time was 101 days.
CONCLUSIONIn this study, talc pleurodesis was safely performed in an outpatient setting with good efficacy and a reasonable complication rate, thereby avoiding hospital admission.
Malignant pleural effusion (MPE) is a frequent and disturbing complication of metastatic disease, and pleurodesis is the procedure that is traditionally used to control MPE.1 Talc is an effective sclerosing agent and has wide international acceptance;1-3 nonetheless, intrapleural administration of talc, which can be done via videothoracoscopy or chest tube, requires hospital admission.3-7 This fact, which is particularly undesirable for patients with advanced disease receiving palliative treatment, has prompted the search for new, less invasive procedures that could be performed on an outpatient basis. This discussion is even more relevant for patients with low performance status (Karnofsky Performance Status [KPS] <70), for whom life expectancy is particularly limited,8-10 reason why they are usually relegated to repeated thoracenteses, a procedure with a high level of recurrence.11 Particularly, for this subgroup of patients, a minimally invasive procedure for the control of MPE that dispenses with the need for hospital admission is of paramount importance. The feasibility of performing pleurodesis on an outpatient basis, through the use of pleural catheters, was suggested in a series of just 10 cases12 and, although it appears to be a good alternative, no further studies have addressed this issue. Therefore, the objective of this study was to evaluate the effectiveness and safety of pleurodesis carried out entirely on an outpatient basis, through talc slurry by pleural catheters in patients with recurrent MPE and KPS ≤70.
MATERIALS AND METHODSFrom January 2005 to July 2007, a prospective trial protocol was conducted in our outpatient Pleural Diseases Unit. The study included patients with the following characteristics: 1) biopsy or cytology proven MPE which was symptomatic and recurrent (according to chest radiographs); and 2) performance status ≤70 (according to the KPS). Patients with the following characteristics were excluded: 1) performance status ≤30 (according to the KPS); 2) Incomplete lung expansion after chest tube drainage (trapped lung) on chest radiograph; 3) social or cognitive conditions that would prevent home care with the drain; 4) hemorrhagic diatheses (platelets <50.000 and INR > 1.5); 5) active skin infection; 6) neoplastic skin infiltration at the drainage site; 7) Previous pleurodesis or chest tube drainage; 8) declining to sign the informed consent.
All patients had their skin examined immediately before the procedure. All of them also had recent laboratory tests (maximum 7 days) prior to catheter insertion. Active skin infection (mainly due to breast cancer) at the site of catheter insertion was diagnosed through physical examination. White blood cells count > 12.000 and fever were findings that supported such a diagnosis. This study protocol was revised and approved by our Institutional Ethics Committee.
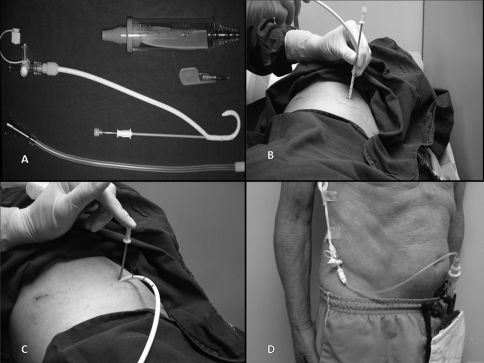
ProcedureEach included patient signed the informed consent approved by our institutional ethics committee. All procedures were carried out in the minor procedures room of our outpatient clinic. In all patients, the skin was prepped with alcoholic clorhexidine and the surgical field isolated with sterile drapes. After local anesthetic (lidocaine 2%) administration, pleural drainage was carried out with 14 Fr pigtail catheters (C-UPTP-1400-WAYNE, Cook Medical, Bloomington, IN) using the trocar technique (Figure 1A-C). After that, a Heimlich valve was connected to the the drain, and its distal end was connected to a collection bag (Figure 1D).
Proper catheter placement was evaluated immediately after the procedure according to adequate pleural fluid evacuation, absence of hemorrhagic events and physical examination. Evaluation was performed by a trained investigator that followed a pre-established protocol, looking for potential complications of catheter placement (respiratory insufficiency, bleeding, pain and cough). If the catheter was working properly, the patient was discharged after 1 hour of observation and radiological exams were considered unnecessary. Before being discharged, the patients received instruction on how to care for the drain.
The first visit after catheter placement was scheduled to the fifth day after the procedure. On the first visit, the patients underwent a chest radiograph; if this showed pulmonary expansion greater than 90% of the affected hemithorax, the patient was selected for pleurodesis, otherwise the patient was discharged and asked to return in 7 days. In this case, a new chest radiograph was performed, similar to what was described previously. If lung expansion was greater than 90%, the patient was elected for pleurodesis. If complete chest expansion did not occur (trapped lung), pleurodesis was not performed and the patient was excluded from the trial. In such patients, the drain was kept in place until the daily output was <100 mL/day.
Patients selected for pleurodesis underwent talc slurry through the pig-tail catheter (3 g of noncalibrated talc (Magnesita Refratarios SA: Contagem, Brazil) in 60 mL of saline solution and 5 mL lidocaine 2%). Systemic analgesia was administered only when necessary. The drain was kept closed for 1 hour; after this, the drain was opened and the patient discharged and instructed to return in 1 week. Complications after pleurodesis were actively searched after talc instillation and in each visit through physical examination and a standardized questionnaire. The drain was removed if the flow was less than 100 mL/day.
After drain removal, a return visit was scheduled to the 30th post catheter insertion day, for clinical evaluation and a new chest radiograph. Thereafter, the visits were each 3 months. On all the return visits, new chest radiographies and the occurrence of complications were evaluated. If the patients were symptomatic and there was evidence of fluid accumulation on the chest radiograph, a thoracentesis was performed. If there was doubt between fluid accumulation and tumor progression or lung infiltration, a chest CT scan was performed. Patients who did not show up for the scheduled consultation were contacted by telephone. Unscheduled visits were arranged in cases of clinical need (recurrences, complications, etc.). Patients were followed until death or until the end of the study in January, 2008.
Data analysisThe variables considered for analysis of clinical effectiveness were symptomatic improvement and MPE recurrence. Symptomatic improvement was evaluated between 7 and 15 days post drainage, with a three-point scale filled out by the patient: complete improvement (absence or minimal symptoms), partial improvement (persistent symptoms but with significant improvement) or no improvement (all the symptoms persist). Recurrence was a binary variable (occurred/did not occur) and was considered to have occurred when, during the clinical follow-up, after removal of the drain, the patient presented recurrence of the symptoms (dyspnea and/or chest pain) associated with radiological evidence of MPE reaccumulation as revealed on the chest radiograph (assessed by two independent investigators). If there was any doubt between fluid reaccumulation and tumor progression, a chest tomography was performed. The recurrence rate was evaluated for the whole sample, and for the patients who remained alive 30 days after pleurodesis.
The following variables were also studied: complications (both after drainage and pleurodesis as well as during all follow-up), drainage time and need for additional pleural procedures. The need for new pleural procedures (thoracentesis, pleural drainage or videothoracoscopy) after removal of the drain was a binary variable (yes/no). Such procedures were indicated when MPE recurrence or pleural complications as empyema or hemothorax were diagnosed by our clinical staff.
Descriptive statistical analysis was used to summarize the characteristics of the studied patients, success rates of the pleurodesis, complications, and the need for new pleural procedures. The impact of nominal factors in the postoperative lung expansion, recurrences and complications was analyzed with the Chi-Square or exact Fisher tests. Logistical regression was used to identify factors associated with mortality within 30 days and with patient survival. All analyses were carried out with a level of significance of p <0.05.
RESULTSFrom January 2005 to July 2007, 147 patients with MPE were seen in our Pleural Diseases Unit and 72 fulfilled the inclusion criteria. The main reason for a patient not be included in the study was KPS>70. Eight, out of these 72 patients were eventually excluded for the reasons discriminated in Figure 2. Therefore, 64 patients were elected for pleural catheter insertion. These patients underwent 66 pleural catheter implantations (2 patients had bilateral effusion). The demographic characteristics of the studied patients are listed in Table 1.
Characteristics of the patients elected for chest drainage.
| Sex (M/F), n | 24/40 |
| Age (years) | 61.4 (39-87) |
| Affected side (R/L/B), n | 34/28/2 |
| Time from diagnosis until pleurodesis (months) | 2 (1-13) |
| Previous Thoracenthesis(n)* | 3 (1-7) |
| Primary Site (n) | |
| Breast | 27 |
| Lung | 22 |
| Undetermined | 7 |
| Kidney | 3 |
| Others** | 5 |
| Oncological Treatment (n) | |
| Thoracic radiotherapy | 14 |
| Chemotherapy (ongoing) | 20 |
| None | 25 |
| Pleural Fluid Characteristics | |
| Oncotic cytology (pos/neg) | 34/10 |
| pH | 7.2 (6.8-7.7) |
| Glucose (mg/dL) | 88 (11-190) |
| Protein (g/dL) | 4.3 (1.7-6.2) |
| DHL (U/L) | 709 (92-6194) |
| ADA (U/L) | 22.1 (9.7-113.6) |
Thoracentesis*: Number of thoracentesis prior to pleurodesis
Others**: Lymphoma, ovarian carcinoma, laryngeal carcinoma.
Results are expressed as median (+range)
No complications were observed during catheter implantation. Fifty-two patients (78.7%) had lung expansion greater than 90% of the affected hemithorax, while 14 patients (21.2%) had incomplete lung expansion (trapped lung), and therefore were excluded from the trial. There was no correlation between the degree of lung expansion and age, sex, primary cancer site or number of previous thoracenthesis. Pleurodesis was performed on the first scheduled return visit to the outpatient clinic in 38 cases (73%) and on the second visit in 14 (27%). The average time between catheter insertion and pleurodesis was 4.2 (1–15) days. No complications were observed immediately after the talc slurry. The average drainage time was 9.9 (4-26) days.
Late complications were observed after 5 (9.6%) procedures. Obstruction occurred in 4 (7.7%) patients and was the most frequently observed complication. In 2 patients the catheters could be unblocked with a flush of saline, and drainage resumed spontaneously. In the other 2 patients this procedure failed, and the catheters were removed. Pleural empyema occurred in 1 (1.9%) patient. Therapy consisted of antibiotic treatment and videothoracoscopy, with satisfactory result. There were no deaths related to the procedure. Bleeding, cellulitis, adult respiratory distress syndrome, air leak and other complications described in the literature did not occur in this series.
Clinical effectiveness results and additional pleural procedures are depicted in table 2. In 4 patients, the analysis of symptomatic improvement could not be carried out due either to death or inability of the patient to come to the consultation due to poor clinical status. Recurrence was observed in 5/52 patients (9.6%) during the whole study period. For the patients that were alive after 30 days, recurrence occurred in 5/36 patients (13.9%). Primary site cancer, time until pleurodesis, previous oncological treatment, and lung expansion did not correlate with the recurrences. Additional procedures were necessary in 6 patients (11.5%); in 5 due to recurrence of the effusion (1 videothoracoscopy, 1 chest tube drainage, 3 thoracenthesis) and in 1 due to pleural empyema (videothoracoscopy). These events were considered as failure of treatment.
Clinical Effectiveness and Additional Pleural Procedures.
| Pleurodesis (n = 52) | ||
| Symptomatic Improvement (n = 48) | Complete | 31 (64.5%) |
| Partial | 17 (35.5%) | |
| No | 0 | |
| Recurrence (30 days) (n = 36) | 5/36 (13.9%) | |
| Additional Pleural Procedures (n = 6) | Videothoracoscopy | 2 (38%) |
| Chest Tube Drainage | 1 (1.9%) | |
| Thoracentesis | 3 (65.7%) |
After the 30th day consultation, eight patients were lost to follow-up and seven of them (patients or their families) could be contacted by telephone, none of these underwent new pleural procedures according to the telephone interview but late chest radiographs of these patients could not be obtained. The median follow-up time for all patients was 60 days. The average survival time after catheter introduction was 101 and days and 11 patients were still alive at the end of the data collection. A Kaplan-Meier curve estimating survival of the included patients is depicted in graphic 1. In this sample, sex (p = 0.58), primary site (p = 0.07), previous oncological treatment (p = 0.29), pleurodesis (p = 0.97), lung expansion (p = 0.69) and symptomatic control (p = 0.1) had no influence on patient survival.
The 14 patients that were submitted to chest tube drainage and were excluded from the protocol (trapped lung patients) were followed in the same manner as those in the trial. Average drainage time was 26.8 (5-94) days. Complications occurred in 7 patients (50%), especially in those with drainage lasting over 21 days. Obstruction occurred in 2 patients (14.3%), empyema in 4 (28.5%) and pain in 1 (7.1%). Complete symptomatic improvement was depicted in 9 patients (69.4%), partial improvement in 2 (15.3%) and no improvement in 2 (15.3%). In 1 patient, symptomatic improvement analysis could not be measured due either to death or inability of the patient to come to the consultation. Due to the high rate of complications in this subset of patients, it is no longer our policy to maintain drainage over 7 days in patients with partial or no lung expansion.
DISCUSSIONIn the present study we observed that pleurodesis may be performed in an outpatient setting safely with good efficacy and reasonable complication rate even in a subgroup of patients with low performance status. The trial protocol was easy to implement and required only adaptation of an already existing wound-care room in our outpatient facility and training of the nursery team on how to instruct patients and their caretakers in the care of the pleural catheter at home. No complications occurred during catheter insertion or during pleurodesis, and the procedures could be carried out comfortably in this outpatient clinic environment. As a minimally invasive procedure, outpatient pleurodesis was easily accepted by patients and referring physicians. Moreover, as hospital admission and operation room scheduling were not necessary, the time from the first consultation at our clinic to the therapeutic procedure shortened.
Our results showed that MPE recurrence occurred in 13.9% of the patients who remained alive 30 days after the procedure, a result comparable with the recurrence rate of 6–28% observed in other pleurodesis series using either videothoracoscopy or chest tube.4-8,13,14 Nonetheless, from an intention-to-treat perspective, 30 (57.6%) procedures out of 52 could be considered successful (i.e., patients were alive 30 days after the procedure with no recurrence and no need of additional procedure). Unfortunately this result reflects general daily practice and this fact was already pointed out by another article15 in which the authors have analyzed pleurodesis outcome from an intention-to-treat perspective and reported a success rate of only 25%, even though they have considered success when patients were free of pleural effusion 60 days after the pleurodesis. Such low success rates reinforce the search for both better predictors and minimally invasive techniques that dispense with hospital admission.
Recently another option for MPE management has been consistently reported, the use of long-term indwelling catheters.16,17 These catheters may be used in an outpatient setting similarly to the procedure we described in the present study. However, no sclerosing agent is administered and patients remain with the catheter for long time and are supposed to periodically drain their own fluid using dedicated vacuum bottles. Interestingly, spontaneous pleurodesis has been observed after a mean time of 90 days in about 70% of patients fit for pleurodesis who underwent long-term pleural catheters insertion.18 Failure in studies addressing long-term indwelling catheters is frequently defined as the need of additional pleural procedures. In our study, additional procedures were necessary in 11.5% of the patients, whether due to MPE recurrence or because of infectious complications, on the other hand in a series of 223 patients who underwent long-term catheter implantation, the need for additional pleural procedures was 9.9%.16 However, these patients remained with the drain in place for an average of 56 days (compared with 9.9 days in the present study). Long-term drainage is a frequent finding in long-term indwelling catheter series and a drawback of such a technique. It was observed that 41.4–45.8% of these patients die with the drain in place, and in those where it is possible to remove the catheter, they remain in the pleural cavity for an average of 29.4–59 days.16,17 Not only long term-drainage but also device availability, costs, and cultural issues - particularly the ability to deal with the device and the willingness to remain with a drain for long periods - are matters of concern.
We observed trapped lung in 21% of our patients and other authors had also failed in identify such features before drainage and found 15 and 20% of patients with trapped lung.4,15 In spite of the fact that such patients had been excluded from the pleurodesis trial, we followed them, and we have found some significant results. From a clinical perspective, the most important finding was the high empyema rate (28.5%) in those who had trapped lung and long-term drainage. In the trapped lung subset of patients, the empyema rate was higher than in those series which long-term indwelling catheters were used.16,17 One possible explanation for this observation might be the fact that pig-tail catheters are not tunneled; therefore,superficial infections would reach the pleural cavity more easily and cause empyema. Therefore, our policy now is to remove chest drains as soon as possible in the event of trapped lung.
The following aspects of this study weaken our conclusions, as a prospective cohort, no comparisons were made with other pertinent techniques, such as pleurodesis by videothoracoscopy or long-term pleural catheters; and, none of the investigators was blinded therefore the number of recurrences and complications might be underestimated. Moreover, talc was the only agent tested and different sclerosing agents as belomicyn, iodopovidone or silver nitrate could provide different results.19,20 Unfortunately, the small number of patients did not enable us to identify risk factors for complications, recurrence, and mortality within 30 days, which would be particularly interesting for specifying those patients who would most benefit from this treatment.
CONCLUSIONSWe can conclude that the strategy used in this study enabled adequate control of pleural effusion symptomatology and was associated with the level of recurrence and the need for additional procedures similar to those of other methods described in the literature. In addition, this technique offers