The purpose of this study was to determine the levels of Cystatin C in healthy term newborns in the first month of life.
INTRODUCTIONCystatin C may be a suitable marker for determining the glomerular filtration rate because it is not affected by maternal renal function.
METHODSCohort study. Inclusion: term newborns with appropriate weight; mother without renal failure or drugs that could affect fetal glomerular filtration rate. Exclusion: malformations; hypertension or any condition that could affect glomerular filtration rate. Cystatin C (mg/L) and creatinine (mg/dl) were determined in the mother (Mo) and in the newborn at birth (Day-0), 3rd (Day-3), 7th(Day-7) and 28th(Day-28) days. Statistics: one way ANOVA and Pearson's correlation tests. Sample size of 20 subjects for α = 5% and a power test = 80% (p<0.05).
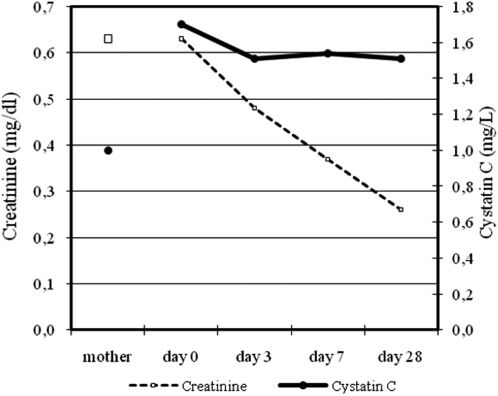
RESULTSData from 21 newborns were obtained (mean ± standard deviation): MoCystatin C = 1.00±0.20; Day-0 Cystatin C 1.70±0.26; Day-3 Cystatin C = 1.51±0.20; Day-7 Cystatin C = 1.54±0.10; Day-28 Cystatin C = 1.51±0.10.
MoCystatin C was smaller than Day-0 Cystatin C (p<0.001), while MoCreatinine was not different from Day-0 Creatinine. Cystatin C only decreased from Day-0 to Day-3 (p = 0.004) but newborns Creatinine decreased along the time. Correlations were obtained between MoCystatin C and MoCreatinine (p = 0.012), as well as Day-3 (p = 0.047) and Day-28 (p = 0.022) Cystatin C and Creatinine values.
CONCLUSIONNeonatal Cystatin C values were not affected by MoCystatin C and became stable from the 3rd day of life.
Newborns have a peculiar renal function and their glomerular filtration rate may vary depending on the degree of renal development at birth.1
In newborns at term, the glomerular filtration rate reaches values below 10% of the adult level, even when considered in relation to body surface or weight. However, the glomerular filtration rate increases progressively to adult values only at 2 years of age.2
In order to detect properly the presence of abnormal renal function, it is crucial to understand the normal value of expected glomerular filtration rate for each gestational and postnatal age, using reliable methods for evaluation.
In general, renal function has been evaluated by the creatinine clearance, which allows the glomerular filtration rate estimation in regular conditions, but can overestimate its value when glomerular filtration rate is less than 2 ml/min/L.2 In the neonatal period, because of the technical difficulty in collecting urine for a few hours on a non invasive procedure, serum creatinine has been used for the evaluation of renal function.3 However, creatinine depends not only on its renal excretion, but also on its production and metabolism. Moreover, at birth, it is a reflection of maternal creatinine and it's not known how long this effect will last.4 In order to find a more precise endogenous marker of glomerular filtration rate, serum cystatin C has been studied.
Cystatin C is a low molecular weight protein, freely filtered by the glomerulus, and almost completely reabsorbed and metabolized in the proximal tubule. It is produced on a constant rate by nucleated cells and its serum concentration depends fundamentally of the glomerular filtration rate.5,6,7 It does not suffer interference of sex, race, height or body composition,8,9 but varies with the age of the individual, being highest at birth, reducing up progressively until 1 year of life,10-13 when reaches levels similar to those of adults. Unlike creatinine, serum cystatin C in the newborn is not influenced by the mother's renal function, 14 which encourages its use to assess the glomerular filtration rate soon after birth.
However, there are not yet standard values defined for this period. This study aims at analyzing the evolution of serum levels of cystatin C in healthy term newborns, during the first month of life.
MATERIALS AND METHODSA cohort study was carried out in the Nursery Annex to the Maternity of Hospital das Clínicas, University of São Paulo, School of Medicine. It was performed in the period from July 1st 2005 to January 31th, 2006. This research project was approved by the Ethics Committee for Review of Research Projects - CAPPesq of the hospital where it was held, on March 24st, 2005.
Newborns were included, according to the following criteria: gestational age between 37 and 416/7 weeks (full term newborns);15,16 birth weight appropriate for gestational age;17 first minute Apgar score equal to or higher than 7; and whose mother had given consent after being adequately informed.
Exclusion criteria were: mothers with renal failure, chronic or pregnancy-induced hypertension and use of drugs that could have interfered with the fetal renal function during pregnancy;18 newborns with major or kidney malformations; first diuresis after 12 hours of life or diuresis between 24 and 72 hours of life less than 1 ml/kg/h, weight loss until the third day of life higher than 10% of the birth weight; hypertension;19 heart or kidney failure; hemolytic disease; jaundice needing phototherapy; infection; need for intravenous hydration or diuretics, vasoactive or any drug use that could interfere with renal function; or inability to collect all the samples as planed.
The newborns included received usual care according to the rules of the neonatal unit. They were submitted in the first three days of life to systematized clinical evaluation, daily, always held by the same researcher which included control of weight, length and blood pressure as described by Zubrow19 (monitor blood pressure noninvasive, oscilometric - the DINAMAP®) and determination of 24 hours diuresis. All outpatients returned to control at 7 and 28 days, and were evaluated clinically by the same person and submitted to the weight, length and blood pressure control.
Blood was obtained for the determination of the laboratory cystatin C and creatinine of the mother at the time of delivery, and in the newborn at birth in the umbilical cord(Day-0), with 3(Day-3), 7 and 28(Day-28) days.
The cystatin C was measured in samples by immunonephelometry, using the Testkit N Latex Cystatin C, code OQNM, manufactured by DADE BEHRING®. The creatinine was determined by the method of Jaffé without desproteinization. All dosages were performed at the Laboratory of Medical Research in Pediatric Clinic of the Department of Pediatrics, University of São Paulo, School of Medicine.
An ultrasound scan of the kidney and urinary tract until the third day of life was done, always by the same person.
Statistical analysisThe sample size was calculated based on the mean serum cystatin C (1.36 ± 0.35 mg/L)20 and creatinine (0.50 ± 0.30 mg/dl)3 values and the objective was to detect differences equal or greater than 0.24 mg/L (18%) for the mean cystatin C concentrations and 0.20 mg/dl (40%) for the creatinine values.
For an α of 5% and a power test (β) of 80%, the sample was calculated on 20 newborns.
The comparisons between groups were made by the paired Student t test or tests of non parametric Wilcoxon Signed Rank Sum Friedman and 2-way ANOVA. The Pearson's correlation coefficient was used for correlation analysis. The significance was set under 5% (p < 0.05).
RESULTS21 newborns were included in the study.
The characterization of the study population can be seen in Table 1. The evolution of weight in the 1st month of life has been as expected (Table 2). Throughout the study period, 13 newborns had jaundice, without the need for phototherapy (61.90%). One newborn had an atrial septal defect and another one a ventricular septal defect, both without repercussions and which were closed in the third month of life. Blood pressure values were in accord with the reference19 (Figura 1).
Characterization of the population (n = 21).
| Mother characteristics | |
| Age in years (mean ± standard deviation) | 29.10 ± 8.20 |
| 1st pregnancy (%) | 28.60 |
| Prenatal care (%) | 100 |
| Cesarean section (%) | 52.40 |
| Newborn characteristics | |
| Gestational age (weeks) | 39.40 ± 0.90 |
| Birth Weight (g) | 3224.30 ± 306.70 |
| Adequate for Gestational Age (%) | 100 |
| Male (%) | 57.10 |
| Exclusive Breastfeeding (%) | 81.00 |
Data presented as mean ± standard deviation or percent (%).
NB blood pressure and post conceptual gestational age according to reference values of Zubrow20.
As for the evaluation of diuresis, the first urination occurred at 4.90 ± 3.90 hours of life, and the mean diuresis in the Day-2 and Day-3 was of 2.10±0.60 and 2.40± 0.70 ml/kg/h, respectively, ranging from 1.30 to 3.70 ml/kg/h. There were no differences between the diuresis in these two days (p = 0.14) (Table 3).
All the ultrasound scans of the newborn kidneys and urinary tracts were normal.
The newborn's cystatin C values showed consistently higher values than mother's cystatin C. The maximum value occurred on the Day- 0, getting their values stable from Day-3 on. In turn, newborn creatinine did not differ from the mother creatinine in the Day-0, and decreased progressively until the Day-28 (table 4; figure 2).
Levels of cystatin C and creatinine.
| Cystatin C (mg/L) | Creatinine (mg/dl) | ||
|---|---|---|---|
| Mother | 1.00 ± 0.20 | 0.63 ± 0.15 | |
| NB | Day-0 | 1.70 ± 0.26 | 0.63 ± 0.15 |
| Day-3 | 1.51 ± 0.19 | 0.48 ± 0.16 | |
| Day-7 | 1.54 ± 0.15 | 0.37 ± 0.10 | |
| Day-28 | 1.51 ± 0.15 | 0.26 ± 0.05 |
Data presented as mean ± standard deviation.
Creatinine: mother X Day-0, p = 0.545; Day-0 X Day-3, p = 0.007; Day-3 X Day-7, p = 0.002; Day-7 X Day-28, p <0.001.
Cistatina C: mother X Day-0, p <0.001; Day-0 X Day-3, p = 0.004; Day-3 X Day-7, p = 0.419; Day-7 X Day-28, p = 0.455.
The maternal cystatin C and creatinine concentrations showed a positive correlation (r = 0.537,p = 0.012) as Day-3(r = 0.437;p = 0.047) and Day-28(r = 0.498;p = 0.022) cystatin C and creatinine values.
DISCUSSIONThe cystatin C comes out as a reliable marker of renal function, particularly in the first week of life. However, there was not yet in the literature determinations of serum levels of cystatin C in healthy newborns, during the neonatal period. In this study, it was possible to verify that the newborn-term exhibit stable levels of cystatin C from the third day of life and that there was not placental passage of the cystatin C. In view of the need for the selection of case series which were the closest possible to the normal, we established a selection based on strict criteria for inclusion. Furthermore, some clinical data were analyzed, such as: weight, urine output, blood pressure and kidneys ultrasound, so that there was greater safety in relation to the normal physiological development of renal function in these newborns.
The included newborns had a weight loss that occurred within the limits of 5 to 10%, considered normal for healthy term newborns.21 The first urination and urinary output have remained within the expected.2 The systolic and diastolic blood pressure were always within the limits considered normal for corrected gestational age, 19 ultrasonography kidneys evaluation showed no morphological alterations and no disturb of thyroid function was detected.
Thus, it was possible to infer a diagnosis of normal renal function of these newborns, which was corroborated by the analysis of creatinine. On Day-0, creatinine was equal to the mother, decreasing gradually to the Day-28. The levels of creatinine were in line with the values considered normal for healthy term newborns.3,10
The mother creatinine was not different from that of the newborn at birth, which was expected, given the known placental passage of creatinine. In turn, the cystatin C of the mother was different from the newborn cystatin C at birth, as has been described by Cataldi , showing no placental passage of the cystatin C.14 The levels of maternal cystatin C and creatinine were consistent with the values described by Cataldi14 and by Babay22 and showed a correlation between them, as expected, since a number of searches already demonstrated the accuracy of cystatin C to estimate the glomerular filtration rate and its correlation with creatinine.10–14,20,23-26
The cystatin C in the newborns were higher on the Day-0, decreasing from birth to the third day and then the levels remained constant, always higher than the maternal ones. Some authors have sought to determine the normal values of cystatin C in term newborns in the neonatal period: Bökenkamp11 - 1.64 to 2.59 mg/L from 0 to 3 days and 1.52 to 2.40 mg/L from 3 to 30 days; Finney10 - 0.81 to 2.32 mg/L from 0 to 3 months; Harmoinen13 - 1.36 to 2.23 mg/L from 0 to 7 days; Bahar20 - 0.69 to 2.43 mg/L in the umbilical cord and 0.78 to 2.40 mg/L to 3 days and Treiber25 - 1.97 ± 0.36 mg/L (1, 38 to 3.23) in the umbilical cord and 1.93 ± 0.33 mg/L (1.28 to 2.66) in the third day. The results obtained here are contained in each of these bands of normal values, which are very large, since the age groups were not always so restricted and the inclusion criteria to ensure normal renal function were not as stringent as those used here.
We observed a positive correlation between the maternal values of creatinine and cystatin C, as expected.14,22 Besides that other positive correlations were obtained on Day-3 and Day-28 between cystatin C and creatinine values. The significance of these results has yet to be proved, as the glomerular filtration rate could not be studied by us through clearances analysis as desirable because of methodological limitations in term newborns.
It's possible that cystatin C could be a reliable indicator of renal function in newborns, especially in the first week of life, for not having placental passage and being stable from the third day of life.
Nevertheless, from the results obtained in this study, we only can indicate these values of cystatin C as reference serum levels for healthy term newborns. New studies are needed in order to evaluate the reliability of newborn cystatin C values as a marker of renal function in the neonatal period.
This work was supported by a grant from FAPESP (Foundation to Aid Research of the State of São Paulo).