We aimed to assess the chemotactic response of endothelial progenitor cells to angiotensin-converting enzyme inhibitors in T2DM patients after acute myocardial infarction, as well as the associated prognosis.
METHODSSixty-eight T2DM patients with acute myocardial infarction were randomized to either receive or not receive daily oral perindopril 4 mg, and 36 non-diabetic patients with acute myocardial infarction were enrolled as controls. The numbers of circulating CD45−/low+CD34+CD133+KDR+ endothelial progenitor cells, as well as the stromal cell-derived factor-α and high-sensitivity C reactive protein levels, were measured before acute percutaneous coronary intervention and on days 1, 3, 5, 7, 14, and 28 after percutaneous coronary intervention. Patients were followed up for 6 months. Chinese Clinical Trial Registry: ChiCTR-TRC-12002599.
RESULTST2DM patients had lower circulating endothelial progenitor cell counts, decreased plasma vascular endothelial growth factor and α levels, and higher plasma high-sensitivity C reactive protein levels compared with non-diabetic controls. After receiving perindopril, the number of circulating endothelial progenitor cells increased from day 3 to 7, as did the plasma levels of vascular endothelial growth factor and stromal cell-derived factor-α, compared with the levels in T2DM controls. Plasma high-sensitivity C reactive protein levels in the treated group decreased to the same levels as those in non-diabetic controls. Furthermore, compared with T2DM controls, the perindopril-treated T2DM patients had lower cardiovascular mortality and occurrence of heart failure symptoms (p<0.05) and better left ventricle function (p<0.01).
CONCLUSIONSThe use of angiotensin-converting enzyme inhibitors represents a novel approach for improving cardiovascular repair after acute myocardial infarction in T2DM patients.
Type 2 diabetes mellitus (T2DM) is an independent predictor of adverse outcomes in patients with acute myocardial infarction (AMI) (1,2). After AMI, diabetic patients have a higher incidence of angina and heart failure, as well as increased mortality (3,4), which is in accordance with severe left ventricular remodeling and impaired cardiac function (5). Endothelial dysfunction of blood vessels has been shown to contribute to vascular complications in T2DM patients. Bone marrow-derived endothelial progenitor cells (EPCs) play an important role in endothelial regeneration and repair (6). However, this function is impaired in diabetic patients and has been demonstrated to participate in the pathogenesis of vascular complications (7). Patients with AMI exhibit increased mobilization of EPCs from bone marrow (8). Once mobilized into circulation, these EPCs home to the ischemic myocardium and may facilitate heart repair (9). However, the beneficial effects of ischemic-induced EPC mobilization are impaired in T2DM patients (10). Therefore, increasing the numbers of circulating EPCs mobilized from bone marrow may improve cardiac function and prognosis in T2DM patients with AMI.
Angiotensin-converting enzyme (ACE) inhibitors are a standard therapy in cardiovascular disease and have been demonstrated to be beneficial to EPCs in some in vitro and clinical studies (11–13). Experimental studies have also revealed that ACE inhibitors can attenuate the development of atherosclerosis-related diseases independent of their vasodilation and hypotensive effects, and this attenuation may be associated with the modulation of EPC mobilization (14). Moreover, in patients with coronary artery disease (CAD) and T2DM, ACE inhibitors have been shown to improve prognosis, although the underlying mechanisms are not fully understood (15). ACE inhibitors increase the expression of many signaling molecules, including stromal cell-derived factor-1α (SDF-1α) and vascular endothelial growth factor (VEGF) (14,16). These molecules are released into circulation from ischemic myocardium and act on the bone marrow to promote the release of EPCs (17,18). We hypothesize that mobilized EPCs may contribute to the beneficial effects of ACE inhibitors on vascular complications in diabetic patients. In the present study, we assessed the functional chemotactic response of EPCs to ACE inhibitors in T2DM patients with AMI, as well as patient prognosis and cardiac function after treatment.
METHODSStudy populationA total of 240 patients with ST-elevation myocardial infarction (STEMI) admitted to our coronary care unit between February 2011 and March 2012 and treated with acute percutaneous coronary intervention (PCI) within 12 h after onset of symptoms were eligible for the present study. We enrolled 68 T2DM patients and 36 non-diabetic (NDM) patients as controls. T2DM was diagnosed as a glycated hemoglobin (HbA1c) level ≥6.1 mmol/l that was controlled by diet or blood glucose-lowering agents. All enrolled patients met the diagnostic criteria for AMI and received successful percutaneous coronary revascularization of the culprit coronary vessel.
The criteria for AMI were as follows: 1) typical ischemic chest pain lasting for ≥30 min, 2) ECG changes representative of new-onset ST-segment elevation ≥0.1 mV in 2 or more contiguous peripheral leads and/or ≥0.2 mV in 2 or more contiguous precordial leads, and 3) evidence of myocardial injury or necrosis as indicated by elevated serum cardiac biomarkers, including creatine kinase (CK) and/or troponin T (TnT).
The exclusion criteria were as follows: 1) blood pressure (BP) ≥130/90 or ≤100/70 mmHg, 2) use of ACE inhibitors or angiotensin receptor blockers (ARBs) during the previous week, 3) any contraindication to ACE inhibitor therapy, 4) severe arrhythmia, 5) level IV cardiac function or left ventricular ejection fraction (LVEF) ≤35%, 6) history of renal or hepatic disorders, and 7) another infarction disease, such as pulmonary or cerebral infarction, in the past 6 months. The 68 T2DM patients were randomized after PCI using a random-number-generating computer system to receive perindopril 4 mg/day (36 patients) or not (32 patients) for 6 months in addition to standard conventional anti-ischemic treatment (including aspirin, clopidogrel, beta blockers, statins, and low-molecular-weight heparin in the first 5 days after PCI). The 36 NDM controls with AMI received no perindopril treatment.
Study ProtocolWe designed a prospective, randomized, open-label, end-point trial that was conducted in accordance with the guidelines of the CONSORT statement (19). The trial is registered with the appropriate authorities (http://www.chictr.org/cn/, #ChiCTR-TRC-12002599). The clinical study protocol followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Nanjing University. Written informed consent was obtained from all enrolled patients.
First, the baseline clinical characteristics of each participant were collected, including risk factors for CAD, blood lipid levels, fasting blood glucose (FBG), glycated hemoglobin, serum cardiac biomarkers, BP, medications, and cardiac structure at admission. Moreover, circulating EPC counts were determined by flow cytometry before acute PCI and on days 1, 3, 5, 7, 14, and 28 after PCI. At the same time points, plasma samples were obtained and stored at −80°C for VEGF, SDF-1α, and high-sensitivity C reactive protein (hsCRP) analysis. All patients visited the hospital for BP and echocardiography examinations at months 1 and 6 after AMI. Cardiovascular events were also investigated during follow-up.
Evaluation of circulating EPCs and plasma VEGF, SDF-1α, and hsCRP in patientsEarly-stage EPCs from bone marrow were characterized as CD45−/low+ mononuclear cells (MNCs) and by the expression of surface CD34, CD133, and the endothelial-specific antigen KDR (20,21). Briefly, 1 ml of blood was drawn, and total MNCs were isolated by density-gradient centrifugation on Ficoll 1077 at 1024 g for 25 min. After washing with phosphate-buffered solution (PBS), 1×106 MNCs were incubated in the dark for 30 min with 5 μl of PE-Cy5.5-conjugated CD45 (Biolegend, San Diego, CA, USA), 5 μl of fluorescein isothiocyanate (FITC)-conjugated CD34 (Biolegend), 5 μl of phycoerythrin (PE)-conjugated CD133 (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), and 10 μl of allophycocyanin (APC)-conjugated KDR (R&D Systems, Minneapolis, MN, USA). Then, 1 ml of 1×Erythrocyte Lysing Solution (Biolegend) was added, and the cells were incubated for 10 min in the dark. Quantitative 4-color flow cytometric analysis was performed using a fluorescence-activated cell sorter (FACS Canto, BD Biosciences, Franklin Lake, NJ, USA), with 100,000 events counted per sample. CD45−/low+CD34+CD133+ KDR+ EPC counts were expressed as the count per 106 MNCs. Plasma concentrations of VEGF, SDF-1α, and hsCRP were measured by an enzyme-linked immunosorbent assay (R&D Systems for VEGF and SDF-1α; Siemens, Erlangen, Germany for hsCRP) according to the manufacturer's instructions.
Patient follow-up to assess cardiac function, BP, and cardiovascular eventsClinical follow-up was performed at an outpatient clinic and by telephone contact. All BP measurements and echocardiography (SONOS 5500, Philips Medical Systems, Best, The Netherlands) were performed by physicians blinded to the patients' profiles and treatment. In addition, cardiovascular events, which indicated the end of study, were also assessed. Endpoints of the study included cardiovascular mortality, re-infarction, re-hospitalization for unstable angina or heart failure, stroke, and death for other reasons. In addition, patients were asked about the symptoms of ischemia and heart failure. Cardiovascular mortality was defined as a documented death due to sudden death or a fatal myocardial infarction, including death due to coronary intervention, congestive heart failure, and other cardiac reasons. Re-infarction was defined as the presence of recurrent clinical symptoms (or the development of new electrocardiographic changes) accompanied by new elevations of CK-MB and TnT levels.
Statistical analysisData are presented as the mean ± SEM for continuous variables and as proportions for categorical variables. We used the chi-square test for comparisons of categorical variables, whereas comparisons of continuous variables were performed using an independent samples t-test or ANOVA with Dunnett's test, as appropriate. For comparison of cumulative mortality rates, we performed a Kaplan-Meier survival analysis and the log-rank t test. In all cases, statistical significance was defined as p<0.05 (two-tailed). All analyses were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
RESULTSPatient characteristicsAs shown in Table 1), all enrolled patients had high TnT and CK-MB levels; FBG and HbA1C levels were higher in patients with T2DM. As expected, the use of oral hypoglycemic agents and/or insulin was more frequent among T2DM patients. Moreover, diabetic patients had slightly higher blood lipid levels, especially low-density lipoprotein (LDL)-cholesterol levels (p<0.05). There was a tendency for worse cardiac function in T2DM patients. Table 2) displays the baseline characteristics of the diabetic patients in the perindopril and control groups. The demographic characteristics and cardiovascular risk profiles were comparable between the two groups at admission. Irrespective of ACE inhibitor therapy, there was no difference in the medication used before and after hospital admission, as well as in cardiac structure and function.
Clinical characteristics of NDM and T2DM patients at admission.
| Baseline characteristics | NDM patients (n = 36) | T2DM patients (n = 68) | p-value |
|---|---|---|---|
| Risk factors | |||
| Male | 28 (78%) | 54 (79%) | 1.00 |
| Age (years) | 64±3 | 66±2 | 0.61 |
| Hypertension, n (%) | 22 (61%) | 40 (59%) | 1.00 |
| Current smoking, n (%) | 24 (67%) | 48 (71%) | 0.76 |
| Hyperlipidemia, n (%) | 2 (6%) | 8 (12%) | 0.65 |
| Family history of coronary heart disease, n (%) | 2 (6%) | 18 (27%) | 0.14 |
| Biological data | |||
| HbA1c (%) | 5.74±0.13 | 7.57±0.19 | <0.01 |
| Fasting glucose (mmol/l) | 5.93±0.22 | 9.37±0.45 | <0.01 |
| Cholesterol (mmol/l) | 4.22±0.16 | 4.76±0.23 | 0.13 |
| Triglycerides (mmol/l) | 1.28±0.18 | 1.62±0.18 | 0.27 |
| LDL-cholesterol (mmol/l) | 2.02±0.11 | 2.58±0.15 | 0.027 |
| HDL-cholesterol (mmol/l) | 1.06±0.13 | 1.00±0.05 | 0.63 |
| Troponin T | 0.80±0.14 | 1.18±0.13 | 0.14 |
| CK-MB (U/L) | 112.7±25.1 | 106.5±18.2 | 0.88 |
| Blood pressure | |||
| SBP (mmHg) | 118.9±3.3 | 121.4±1.3 | 0.39 |
| DBP (mmHg) | 73.8±2.1 | 74.1±0.8 | 0.92 |
| Medication at admission | |||
| Anti-coagulants | 2 (6%) | 12 (18%) | 0.40 |
| Statins | 0 (0%) | 4 (6%) | 0.54 |
| Beta-blockers | 2 (6%) | 0 (0%) | 0.35 |
| Calcium-channel blockers | 8 (22%) | 24 (35%) | 0.53 |
| Oral hypoglycemics | 0 (0%) | 38 (56%) | <0.01 |
| Insulin | 0 (0%) | 8 (12%) | 0.29 |
| Medication at discharge | |||
| Anti-coagulation therapy | 36 (100%) | 68 (100%) | 1.00 |
| Statins | 36 (100%) | 68 (100%) | 1.00 |
| Beta-blockers | 32 (89%) | 52 (77%) | 0.46 |
| Calcium-channel blockers | 0 (0%) | 0 (0%) | 1.00 |
| Oral hypoglycemic agents | 0 (0%) | 30 (44%) | <0.01 |
| Insulin | 0 (0%) | 34 (50%) | <0.01 |
| Echocardiography | |||
| LAD (cm) | 3.87±0.10 | 4.12±0.08 | 0.063 |
| LVDd (cm) | 5.28±0.11 | 5.47±0.07 | 0.15 |
| EF (%) | 49.4±1.35 | 45.9±1.32 | 0.13 |
HbA1c: hemoglobin A1c; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CK-MB: Creatine Kinase-MB; SBP: systolic blood pressure; DBP: diastolic blood pressure; LAD: left atrium diameter; LVDd: left ventricular diastolic diameter; EF: ejection fraction.
Clinical characteristics of diabetic patients at admission.
| Baseline characteristics | T2DM patients | p-value | |
|---|---|---|---|
| Perindopril (n = 36) | Control (n = 32) | ||
| Risk factors | |||
| Male, n (%) | 26 (72%) | 28 (88%) | 0.41 |
| Age (years) | 66±3 | 66±2 | 0.93 |
| Hypertension, n (%) | 22(61.1%) | 18 (56.3%) | 1.0 |
| Current smoking, n (%) | 24 (66.7%) | 24 (75%) | 0.72 |
| Hyperlipidemia, n (%) | 6 (16.7%) | 2 (6.3%) | 0.60 |
| Family history of coronary heart disease, n (%) | 10 (27.8%) | 8 (25%) | 1.0 |
| Biological data | |||
| HbA1c | 7.37±0.29% | 7.83±0.30% | 0.33 |
| Fasting glucose (mmol/l) | 9.73±0.69 | 8.76±0.77 | 0.34 |
| Cholesterol (mmol/l) | 4.77±0.21 | 4.76±0.50 | 1.0 |
| Triglycerides (mmol/l) | 1.63±0.30 | 1.60±0.25 | 0.94 |
| LDL-cholesterol (mmol/l) | 2.59±0.14 | 2.58±0.32 | 0.98 |
| HDL-cholesterol (mmol/l) | 0.98±0.07 | 1.03±0.09 | 0.68 |
| Troponin T | 1.10±0.19 | 1.30±0.21 | 0.61 |
| CK-MB (U/l) | 101.0±25.5 | 112.8±29.1 | 0.84 |
| Blood pressure | |||
| SBP (mmHg) | 123.5±1.9 | 120.9±2.2 | 0.49 |
| DBP (mmHg) | 74.7±1.1 | 77.9±1.2 | 0.15 |
| Medication at admission | |||
| Anti-coagulation therapy | 8 (22.2%) | 4 (12.5) | 0.66 |
| Statins | 2 (5.6%) | 2 (6.3%) | 1.0 |
| Beta-blockers | 0 (0%) | 0 (0%) | 1.0 |
| Calcium-channel blockers | 14 (38.9%) | 10 (31.3%) | 0.73 |
| Insulin | 6 (16.7%) | 2 (6.3%) | 0.60 |
| Medication at discharge | |||
| Anti-coagulation therapy | 36 (100%) | 32 (100%) | 1.0 |
| Statins | 36 (100%) | 32 (100%) | 1.0 |
| Beta-blockers | 28 (77.8%) | 24 (75%) | 1.0 |
| Calcium-channel blockers | 0 (0%) | 0 (0%) | 1.0 |
| Insulin | 16 (44.4%) | 18 (56.3%) | 0.73 |
| Echocardiography | |||
| LAD (cm) | 4.04±0.08 | 4.22±0.15 | 0.28 |
| LVDd (cm) | 5.44±0.11 | 5.52±0.08 | 0.58 |
| EF (%) | 47.1±1.68 | 44.5±2.47 | 0.38 |
HbA1c: hemoglobin A1c; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CK-MB: Creatine Kinase-MB; SBP: systolic blood pressure; DBP: diastolic blood pressure; LAD: left atrial diameter; LVDd: left ventricular diastolic diameter; EF: ejection fraction.
Figure 1 shows the changes in circulating CD45−/low+CD34+CD133+KDR+ EPC counts over time. In the NDM patients, circulating EPCs were increased on day 1 after PCI and reached a peak on day 5, and these counts were significantly higher than the counts in the diabetic groups at the same time points (day 1: NDM controls: 90±13/106 MNCs vs. T2DM controls: 43±7/106 MNCs vs. treated T2DM: 46±10/106 MNCs, p<0.05 between the NDM and T2DM groups; day 5: NDM controls: 154±24/106 MNCs vs. T2DM controls: 64±9/106 MNCs vs. treated T2DM: 91±19/106MNCs, p<0.05 between the NDM and two T2DM groups). In patients with T2DM, the EPC peak was delayed to day 7, and the count was decreased in the T2DM controls (103±23/106 MNCs). However, in perindopril-treated T2DM patients, the EPC counts steadily increased from days 1 to 7 after AMI and were approximately 2.5-fold higher than the T2DM control counts on day 3, finally reaching the same level at a later time (day 7) compared with the peak of the NDM controls (day 5). Moreover, half a month after AMI, when circulating EPC counts fell quickly in all patients, counts in the perindopril group were still higher (67±11/106 MNCs) than those in controls (44±13/106 MNCs in the T2DM controls and 52±7/106 MNCs in the NDM controls, p<0.05).
Changes in circulating CD45−/low+CD133+CD34+KDR+ EPCs in NDM and T2DM patients with AMI. The EPC counts in the peripheral blood were determined by flow cytometry before acute PCI and on days 1, 3, 5, 7, 14, and 28 after PCI. (A–C) Flow cytometric analysis of EPCs for each group. (A) CD45−/low+ EPC subset analysis was conducted on the Gate1 region using PerCP-Cy5.5-labeled antibodies against CD45. (B) The proportion of CD34+ cells was analyzed in the Gate2 region by flow cytometry using FITC-labeled antibodies against CD34. (C) The proportion of CD133+KDR+ cells was analyzed in the F2 region by two-color flow cytometry using PE-labeled antibodies against CD133 and APC-labeled antibodies against KDR. (D) EPC kinetics over time for all three groups. a: p<0.05 vs. the T2DM controls at the same time point; b: p<0.05 vs. the perindopril-treated T2DM group at the same time point; c: p<0.05 vs. the NDM controls at the same time point; d: p<0.05 vs. the T2DM controls at the peak point.
Before PCI, plasma VEGF and SDF-1α levels were decreased in diabetic patients compared with the NDM subjects. Although the kinetics of VEGF over time were similar between NDM and T2DM controls, an apparent impairment in the kinetics on day 1 and day 5 (peak time) was observed in the T2DM controls. Perindopril treatment increased plasma VEGF from days 3 to 5, reaching a peak on day 7, which was much higher than the peak observed in the T2DM controls (263.85±39.75 vs. 147.24±21.32 ng/l, p<0.05) and similar to the peak of the NDM controls (209.48±29.68 ng/l) (Figure 2A). Regarding plasma SDF-1α levels, the NDM controls had the highest SDF-1α level on day 5, but there was no significant difference in this level compared with that in the T2DM groups at the same time point. A notable decrease was observed on days 3 and 7 in the T2DM controls, but the SDF-1α levels were increased by perindopril on day 7 (peak point) (treated T2DM: 2542.5±103.7 ng/l on day 7 vs. NDM controls: 2114.9±191.7 ng/l on day 5 vs. T2DM controls: 1784.7±65.0 ng/l on day 5, p<0.05 between the treated T2DM and two control groups at the peak point) (Figure 2B). In addition, the T2DM controls exhibited a significant increase in plasma hsCRP levels from days 1 to 7, with a peak on day 3 after AMI. The changes in hsCRP levels in the NDM controls were almost the same as those in the T2DM controls, but the levels were much lower at all time points in the NDM controls. The beneficial effect of perindopril on this parameter was observed from day 3 (treated T2DM: 13.69±2.86 mg/l vs. NDM: 23.84±4.18 mg/l vs. T2DM controls: 78.50±17.40 mg/l, p<0.05 between the T2DM controls and the other two groups on day 3) and constantly decreased thereafter (Figure 2C).
Changes in plasma VEGF, SDF-1α, and hsCRP levels in NDM and T2DM patients with AMI. Plasma samples were obtained from 1 ml of peripheral blood before acute PCI and on days 1, 3, 5, 7, 14, and 28 after PCI. VEGF, SDF-1α, and hsCRP analyses were performed by an enzyme-linked immunosorbent assay. (A, B, and C) Plasma VEGF, SDF-1α, and hsCRP levels, respectively. a: p<0.05 vs. the T2DM controls at the same time point; b: p<0.05 vs. the treated T2DM group at the same time point; c: p<0.05 vs. the NDM controls at the same time point.
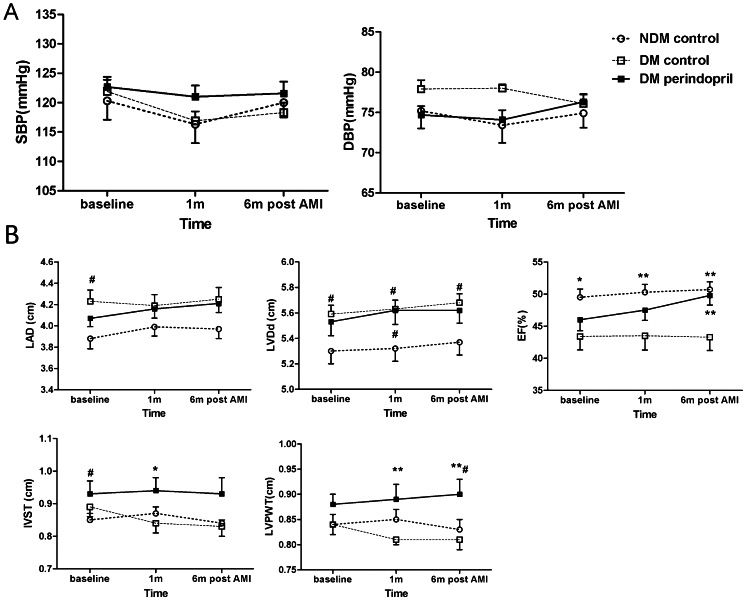
No patient was lost to follow-up during the 6-month period. There was no significant change in BP over time in the three groups (Figure 3A). The T2DM patients who did not receive ACE inhibitor treatment exhibited an increased left ventricular end-diastolic diameter (LVDd) and a decreased left ventricular EF (LVEF) at admission and follow-up compared with the NDM patients. The effect of perindopril on LVDd was limited, but LVEF was improved after 6 months of perindopril treatment (treated T2DM: 49.8±1.5% vs. NDM controls: 50.7±1.2% vs. T2DM controls: 43.3±2.1%, p<0.01 between the T2DM controls and the other two groups). There was no difference in left atrial diameter (LAD) and inter-ventricular septal thickness (IVST) between the three groups, but a change in the left ventricular posterior wall thickness (LVPWT) was observed in the T2DM patients receiving perindopril treatment. ACE inhibitor-treated subjects had an increased LVPWT over time, especially by month 6 (treated T2DM: 0.90±0.03 cm vs. T2DM controls: 0.81±0.02 cm vs. NDM controls: 0.83±0.02 cm, p<0.05 between the treated T2DM group and both control groups) (Figure 3B).
Changes in blood pressure and cardiac function over time in NDM and T2DM patients after AMI. (A) Systolic and diastolic blood pressure; (B) Cardiac function examined by echocardiography, shown are LAD, LVDd, EF, IVST, and LVPWT. #p<0.05 vs. the NDM controls, ∗p<0.05 vs. the T2DM controls, ∗∗p<0.01 vs. the T2DM controls.
There were no deaths in the NDM control and perindopril-treated T2DM groups. The T2DM patients who did not receive ACE inhibitors had a higher mortality rate during follow-up (18.75% vs. 0%). Four of the patients died from acute congestive heart failure (two at 2 weeks, one at 5 weeks, and one at 8 weeks). Of the other two deaths, one was due to sudden death at 3 weeks, and the other was caused by a fatal subarachnoid hemorrhage 4 weeks post-AMI. No patient suffered a stroke or re-infarction. The control T2DM patients had higher rates of ischemia symptoms and re-hospitalization for unstable angina and heart failure, but the rate difference did not reach statistical significance. Most importantly, a higher proportion of T2DM controls compared with treated T2DM patients experienced heart failure symptoms (T2DM controls: 56.25% vs. treated T2DM: 16.67%, p<0.05) (Table 3).
Cardiovascular events during the 6 months of follow-up.
| Clinical outcomes | NDM controls | T2DM patients | Unadjusted hazard ratio between diabetics | p-value | |
|---|---|---|---|---|---|
| Perindopril | Control | ||||
| Cardiovascular mortality | No | No | 25% | – | – |
| Death for other reasons | No | No | 6.25% | – | – |
| Re-infarction rate | No | No | No | – | – |
| Nonfatal stoke | No | No | No | – | – |
| Re-hospitalization for unstable angina and heart failure | 5.56% | 11.11% | 25% | 2.25 | 0.39 |
| Ischemia symptoms | 16.67% | 16.67% | 25% | 1.5 | 0.68 |
| Heart failure symptoms | 11.11% | 16.67% | 56.25% | 3.37 | <0.05 |
In this study, we observed that the circulating EPC levels were decreased in T2DM patients with AMI and that their peak level was delayed compared with that in NDM patients. These observations are in accordance with the observed reduced plasma VEGF and SDF-1α levels, increased plasma hsCRP level, and worse cardiac function and prognosis in T2DM patients compared with NDM patients. However, this impaired mobilization of bone-marrow EPCs was improved by ACE inhibition because perindopril-treated T2DM patients had an earlier and greater increase in bone-marrow EPCs compared with T2DM controls, which lasted up to 14 days post-AMI. Moreover, plasma VEGF, SDF-1α, and hsCRP levels were also improved. Furthermore, perindopril-treated T2DM patients suffered a lower frequency of cardiovascular events, as demonstrated by a decreased cardiovascular mortality and occurrence of heart failure symptoms together with improved LVEF and left ventricular posterior wall hypertrophy.
Based on our results, increases in EPC counts due to perindopril administration in T2DM patients may play a pivotal role in improving prognosis after AMI. Because vessel endothelial cell injury induced by T2DM is regarded as the main reason for composite cardiovascular diseases, EPCs activated by perindopril could contribute to ongoing endothelial repairs by providing a circulating pool of cells that can home to injured parts of the artery or replace dysfunctional endothelial cells (22,23). Additionally, EPCs, once mobilized into the peripheral circulation, locate to the infarcted myocardium and are involved in the formation of new vessels (24,25), which will then improve the blood supply in ischemic penumbra, finally leading to decreased infarction area, improving LV function and reducing LV remodeling after AMI (26). As suggested by our results at even half a month after AMI, the numbers of circulating EPCs were significantly higher in the perindopril-treated T2DM group, contributing to a better EF and a decreased occurrence of heart failure symptoms.
Many studies suggest that ACE inhibition is an effective approach for the treatment of cardiovascular diseases in T2DM patients and in T2DM-associated vascular complications, but the underlying mechanisms are not clearly understood (27,28). In our study, the significant EPC activation observed may be partly associated with the different effects of the ACE inhibitors themselves, including inhibiting bradykinin degradation, increasing cGMP in vascular endothelial cells, restraining the renin-angiotensin system (RAS) activity, and decreasing the production of oxygen free radicals, which leads to sensitized NO synthase (NOS) and increased NO release. All these effects can in turn induce EPC mobilization from the bone marrow (29).
Furthermore, it has already been reported that circulating EPCs mainly originate from the bone marrow and that this mobilization involves a complex interaction between a number of cytokines and active molecules, such as the hypoxia inducible factor-1α (HIF-1α), VEGF, and SDF-1α. These factors could participate in the regulation of EPC mobilization from the bone marrow and homing to the ischemic myocardium. Moreover, previous studies have also indicated that VEGF may be involved in ACE inhibitor-induced restoration of the myocardial structure (16,30,31). We observed that after taking perindopril, plasma VEGF and SDF-1α levels rose in post-AMI T2DM patients, and the kinetics of these two proteins were very similar to the kinetics of the EPCs. Increased plasma VEGF can mobilize EPCs from the bone marrow for peripheral circulation through the activation of the KDR/Akt/eNOS pathway (32). SDF-1α also plays a vital role in EPC mobilization and homing. It has been reported that the insufficient release of SDF-1α may lead to defective mobilization of EPCs in diabetic rats (33). The only receptor of SDF-1α, CXCR4, is highly expressed on the surface of CD34+ hemopoietic stem cells/progenitor cells. SDF-1α can then induce EPC chemotaxis in ischemic tissue via activation of the HIF pathway (18).
As a prototype marker of inflammation, CRP is now widely used to predict cardiac events in various cardiovascular diseases (34). In T2DM, insulin resistance is constantly associated with continuous, low-level inflammation, leading to increased serum hsCRP levels that finally results in impaired EPC levels and function (35). Recently, CRP has been shown to down-regulate endothelial NOS expression and to promote EPCs apoptosis through modulation of the receptor for advanced glycation end-products (RAGE) (36). Our previous study also demonstrated that following AMI, T2DM patients had low circulating EPC levels and increased plasma hsCRP levels, which may be due to the greater inflammation condition caused by more severe ischemia in T2DM. In addition, ACE inhibitors, as anti-inflammatory agents, have been found to decrease plasma hsCRP levels in patients with CAD (37). In the present study, our data revealed that post-AMI serum hsCRP levels were decreased by perindopril, thus decreasing inflammation, which may be involved in improving ischemia or hypoxia conditions.
Study limitationsThe present study has several limitations. First, because this was a single-center parallel controlled study with many exclusion criteria, this study had a small sample size of 104 subjects, and the results require further confirmation in larger cohorts. Second, the follow-up time was insufficient to observe cardiovascular events, especially congestive heart failure. However, the 6-month post-AMI period is the most important period for cardiac repair and cardiac function recovery. Third, in addition to circulating EPC counts, EPC function may also contribute to cardiovascular repair, but function was not measured in this study.
In this study, we demonstrated that perindopril treatment increased circulating EPC numbers from day 3 in post-AMI T2DM patients, and this increase lasted for at least half a month. These changes were accompanied by a similar increase in plasma VEGF and SDF-1α levels and a decrease in serum hsCRP levels. As a result, higher levels of circulating EPCs may exert beneficial effects on cardiac function and clinical outcomes in T2DM patients. EPCs represent a novel treatment for post-AMI cardiovascular repair in T2DM patients.
AUTHOR CONTRIBUTIONSSun JY planned the study, selected patients, measured circulating EPC counts, performed the statistical analyses, and was involved in the preliminary writing of the manuscript. Zhai L was responsible for patient selection, clinical data collection, and patient follow-up. Li QL conducted the laboratory analyses and patient follow-up examinations. Ye JX was involved in the detection of circulating EPCs. Kang LN contributed to the study design and interpretation of data. Ye JX was involved in writing the manuscript. Xu B supervised the project and was involved in writing the manuscript. All the authors read and approved the final version of the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81070195, 81000055), the Jiangsu Key Laboratory for Molecular Medicine of Nanjing University (Research Grant 2008), and the Natural Science Foundation of Jiangsu (grant numbers GBK2009036, BK2010107). We thank Song Jie, Huang Wei, and Zhang Jing-mei for drawing blood before acute PCI and Liu Yong and Xue Jin-kui for conducting the flow cytometry analysis.
No potential conflict of interest was reported.