To determine the impact of supplemental zinc, vitamin A, and glutamine alone or in combination on growth, intestinal barrier function, stress and satiety-related hormones among Brazilian shantytown children with low median height-for-age z-scores.
METHODS:A randomized, double-blind, placebo-controlled trial was conducted in children aged two months to nine years from the urban shanty compound community of Fortaleza, Brazil. Demographic and anthropometric information was assessed. The random treatment groups available for testing (a total of 120 children) were as follows: (1) glutamine alone, n = 38; (2) glutamine plus vitamin A plus zinc, n = 37; and a placebo (zinc plus vitamin A vehicle) plus glycine (isonitrogenous to glutamine) control treatment, n = 38. Leptin, adiponectin, insulin-like growth factor (IGF-1), and plasma levels of cortisol were measured with immune-enzymatic assays; urinary lactulose/mannitol and serum amino acids were measured with high-performance liquid chromatography. ClinicalTrials.gov: NCT00133406.
RESULTS:Glutamine treatment significantly improved weight-for-height z-scores compared to the placebo-glycine control treatment. Either glutamine alone or all nutrients combined prevented disruption of the intestinal barrier function, as measured by the percentage of lactulose urinary excretion and the lactulose:mannitol absorption ratio. Plasma leptin was negatively correlated with plasma glutamine (p = 0.002) and arginine (p = 0.001) levels at baseline. After glutamine treatment, leptin was correlated with weight-for-age (WAZ) and weight-for-height z-scores (WHZ) (p≤0.002) at a 4-month follow-up. In addition, glutamine and all combined nutrients (glutamine, vitamin A, and zinc) improved the intestinal barrier function in these children.
CONCLUSION:Taken together, these findings reveal the benefits of glutamine alone or in combination with other gut-trophic nutrients in growing children via interactions with leptin.
Malnutrition is highly prevalent among children under five years old in developing regions around the world (1). Under-weight, defined by weight-for-age z-scores less than -2 SD (reflecting acute malnutrition), affects 10% of these children, which amounts to approximately 55 million children worldwide (1). Stunting, defined by height-for-age z-scores of less than -2 SD (reflecting chronic malnutrition), affects 31% of these children, or approximately 170 million (1). Wasting, defined by a weight-for-height-for-age z score of less than -2 SD, affects 27% of children and is a condition resulting from stunting, wasting, or both (2). Childhood and maternal malnutrition are responsible for 21% of the global disability-adjusted life years (DALYs) and 2.2 million deaths annually (2).
The global extent of glutamine and arginine deficiency is not well known, although one study has reported that even nourished children or only mild-to-moderate undernourished children often have low plasma concentrations of both of these amino acids (3). Furthermore, deficiencies of vitamin A and zinc are estimated to result in 0.6 and 0.4 million deaths, respectively, and account for a potential total of 9% of global childhood DALYs (2,4).
Children with heavy enteric diseases and diarrheal burdens early in life often suffer from life-threatening malnutrition and growth retardation. Those children who survive to later childhood or adulthood tend to have poor physical fitness and reduced academic and work accomplishments, which are associated with compromised cognitive development, and for women, offspring with lower birth weights (5).
Hormone levels in growing children, such as leptin, insulin-like growth factor-1 (IGF-1), adiponectin, and cortisol, are affected by nutritional factors, daily diet, or both during childhood. Leptin is produced primarily in the adipose tissue and is positively associated with body weight, body fat mass, and recent energy intake (6). In non-obese persons, increased serum concentrations of leptin are associated with decreased appetite (6). Adiponectin, like leptin, is also produced by adipose tissue. However, in contrast to leptin, serum concentrations of adiponectin are lower in obesity and higher in individuals with lower body fat mass (7). IGF-1 is an insulin-like hormone released by the liver that reflects the hepatic anabolic-catabolic balance and is increased by both caloric and protein intake (8). Thus, IGF-1 is a useful biomarker for assessing nutritional status (9). In contrast, cortisol levels are increased in malnutrition (10). These hormones have interrelated effects on human growth: leptin potentiates the action of IGF-1, whereas glucocorticoids (such as cortisol) decrease IGF-1 expression, interfere with IGF-1 signaling, and induce IGF-1 resistance (11). In addition to their effects on human somatic growth, these hormones may also affect immune responses. Adiponectin and cortisol are associated with decreased pro-inflammatory responses, whereas leptin and IGF-1 are associated with an increased inflammatory state, which may represent a compensatory adaptation to tissue recovery (12). Nutrient tissue tropism, gut function, and growth have critical relationships with variations in hormonal levels, although these relationships have only recently emerged as an important research area with potential relevance for effective nutritional interventions (13).
Nutrients such as zinc and vitamin A potentially benefit the catch-up growth following enteric infections and diarrhea (14). In an animal study performed by our group, zinc and glutamine improved behavior and growth in undernourished mice during suckling (15). This improved health status following intervention may be reflected by increases in anabolic hormonal levels, such as IGF-1, reflecting systemic improvements in physical growth and fat mass. Zinc is known to increase serum levels of IGF-1 and leptin (16). Retinoids may increase IGF-1 synthesis in the liver and aggravate the detrimental effects of corticosteroids on IGF-1 activity (17).
Glutamine, vitamin A, and zinc are known to enhance gut barrier function by potentially improving nutrient absorption; therefore, they may benefit growth development (3,18). In fact, our group showed that oral administration of a glutamine derivative, alanyl-glutamine, improved both the intestinal barrier and weight gain in children living in an urban community in Fortaleza, CE, Brazil (3). Thus, we hypothesized that glutamine supplementation alone or in combination with vitamin A and zinc would have a positive impact on the intestinal barrier function and consequently trigger growth improvements in young children (3). These associations would be positively correlated with increased plasma leptin and IGF-1 levels and negatively correlated with cortisol and adiponectin. In addition, we investigated whether the combination of glutamine with zinc and vitamin A (two critical gut-trophic nutrients) would additively improve the aforementioned parameters compared with glutamine alone.
MATERIALS AND METHODSEthicsThe study was approved by the Institutional Review Boards (IRBs) of the Federal University of Ceará (UFC), Fortaleza, Ceará, Brazil, and the University of Virginia. Informed consent was obtained from all participants.
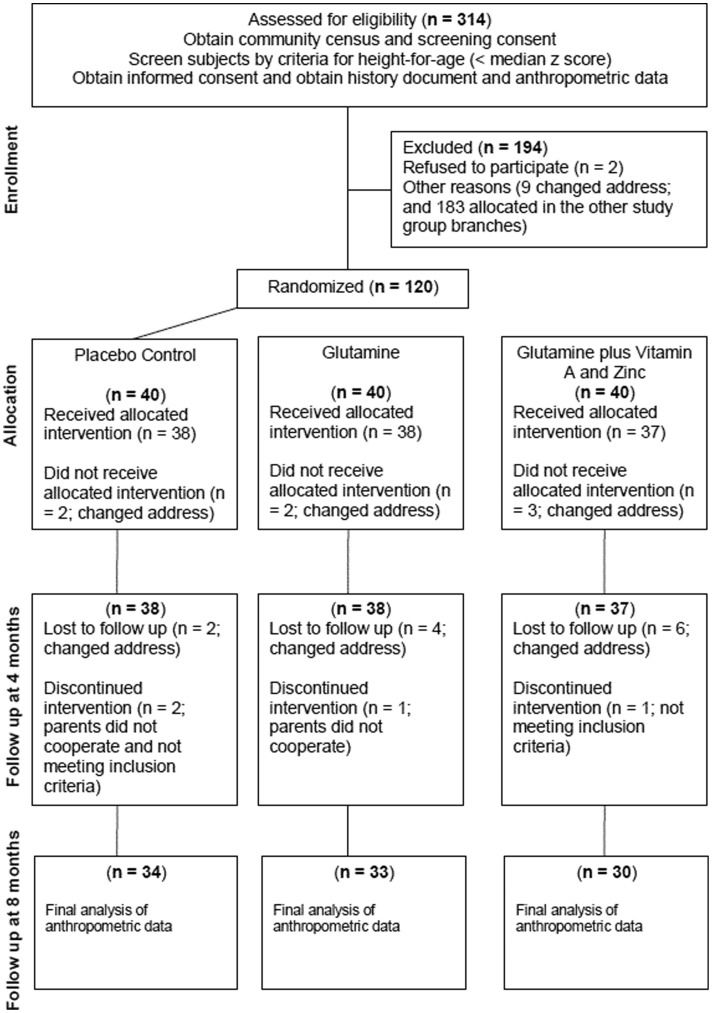
Environment and populationThe study population is located in the Parque Universitário community (3°44'58.27“ south and 38°34'30.80” west) in Fortaleza, Ceará, northeast Brazil, which is 5 km distant from the Clinical Research Unit and Institute of Biomedicine/Center for Global Health (www.upcibimed.ufc.br) laboratories in Fortaleza. Fortaleza has an estimated population of 2.6 million inhabitants and an infant mortality rate of 35 deaths per 1,000 live births. A 1998 census of Parque Universitário revealed a total population of 3,541 inhabitants, of which 957 (27%) were children under the age of 9. The children in this study were a subset of a larger cohort residing in historically endemic settings of enteric and diarrheal illnesses and were thereafter enrolled in a randomized, double-blind, community-based trial to examine the effects of glutamine, vitamin A, and zinc on intestinal barrier function and physical growth. Children aged three months to nine years were initially identified by a community census and screened for height or length measurements. Children were selected if they met the following inclusion criteria: (a) height (or length)-for-age z-scores (HAZ) less than the median for this population (-0.06 z-score), (b) community residency, and (c) parental or guardian consent. The exclusion criteria were as follows: (a) no sibling enrolled in this or any other study in the last two years, (b) a fever >38°C at time of enrollment, and (c) a recognizable chronic condition affecting growth, such as celiac disease, tuberculosis, or any clinically identifiable chronic disease. Figure1 shows a chart of the subjects enrolled in the study protocol. The treatment protocol is summarized in Table1.
Flow of micronutrient supplementation and biochemistry blood testing to study growth, intestinal barrier function, stress and satiety related hormones in Brazilian shantytown children from Fortaleza, northeastern, Brazil, during June 2000 - August 2004, with follow-up between June 2000 and December 2007.
Characteristics of children at baseline, including age, sex, anthropometric indices, intestinal permeability, and treatment group.
| Parameters | Controla)(n = 38) | Glutamineb)(n = 38) | Glutamine + Vitamin A + Zincc)(n = 37) | Total(n = 113) |
|---|---|---|---|---|
| Age(months; median and range) | 47 (6.77-98.75) | 40 (7.65-104.76) | 48 (4.86-99.01) | 45 (4.86-104.76) |
| Sex (male; n [%]) | 20 (53) | 19 (50) | 20 (54) | 59 (52) |
| Z-Score (mean±sem) | ||||
| Weight-for-age | -0.985±0.1856 | -0.500±0.1401 | -0.671±0.1786 | -0.719±0.0993 |
| Height-for-age | -1.158±0.1482 | -0.654±0.1401 | -0.925±0.1626 | -0.913±0.0889 |
| Weight-for-height | -0.187±0.2371 | -0.025±0.1291 | -0.024±0.2008 | -0.080±0.1124 |
| Lactulose:mannitol ratio(median and range) | 0.0810.005-1.125 | 0.0650.001-1.390 | 0.1300.016-1.123 | 0.0830.001-1.390 |
| Adiponectin (ng/ml)(median and range) | 9.4018.268-10.233 | 9.3707.920-10.419 | 9.8348.948-10.807 | 9.5867.920-10.807 |
| Cortisol (ng/ml)(median and range) | 2.8001.429-3.726 | 2.8381.346-4.196 | 2.9862.049-3.843 | 2.8701.346-4.196 |
| IGF-1 (ng/ml)(median and range) | 3.3462.293-5.078 | 3.3962.240-5.474 | 3.6831.892-4.718 | 3.5741.892-5.474 |
| Leptin (pg/ml) | 7.2426.692-8.375 | 6.8825.895-9.333 | 6.9406.175-9.205 | 7.0015.895-9.333 |
There were no significant differences between the control group and the treatment groups (p>0.05).
The intervention protocol was performed as follows:
This study is a prospective, double-blind, randomized, placebo-controlled trial and is registered as a clinical trial (NCT00133406) at the US National Library of Medicine (http://www.clinicaltrials.gov). Enrollment/recruitment was conducted from June 2000 until August 2004. After parental consent was obtained, an experienced field study team (1 nurse and 2 community health workers) interviewed parents and children to collect demographic information, including age, gender, birth weight, level of maternal education, family income, and baseline anthropometric information. A consultant statistician assessed the study design and power. The participants and the study team were blinded to the intervention assignment. Demographic and anthropometric data, information on adverse events and serious adverse events, and laboratory data were collected in case report forms at the field area and at laboratories located at the INCT-Biomedicine, UFC. The adverse event and serious adverse event recording and reporting system were developed and monitored by the National Institutes of Health (NIH), the National Institute of Allergy and Infectious Diseases (NIAID), and the Division of Microbiology and Infectious Diseases (DMID).
Nutritional intervention and surveillanceA total of 314 children were randomized with respect to receiving vitamin A (100,000 IU retinyl palmitate if <12 months old or 200,000 IU retinyl palmitate if ≥12 months old every four months), which was given in one dose at 0, 4, and 8 months into the study protocol; zinc, which was given twice weekly at a dose of 40 mg; or both vitamin A and zinc. The study was conducted for 1 year, and half of each group received glutamine (16-g daily dose given over ten days starting at the first month of the study protocol) (22). A total of 167 children were available and eligible for cognitive testing. Trained health care agents working on the study surveillance team administered the micronutrients. The health care workers administered the micronutrients during home visits with direct observation of intake.
L-Glutamine was obtained from Rexim (Courbevoie, France); L-glycine and zinc acetate were obtained from Spectrum Chemicals (Gardena, CA, USA); vitamin A (retinyl palmitate in vegetable oil with 40 IU of alpha-tocopherol as an antioxidant) was obtained from Hoffman-La Roche, Basel, Switzerland. Isomolar glycine (8.3 g/daily) was used as a placebo for glutamine, Tanjal juice was used as a placebo for zinc, and the same amounts of alpha-tocopherol and vegetable oil were used as a placebo for vitamin A. Tocopherol was chosen as a placebo for vitamin A because it is also a fat-soluble vitamin and the tocopherol dosing preparation is similar to that of vitamin A (capsule dosing). The color, size, and taste were the same between vitamin A and placebo capsules.
A computer-generated random number list was used to assign children to one of eight treatment arms: 1) Placebo; 2) Glutamine; 3) Zinc; 4) Vitamin A; 5) Glutamine + Zinc; 6) Glutamine + Vitamin A; 7) Zinc + Vitamin A; or 8) Glutamine + Zinc + Vitamin A. The treatment group and the oral treatment regimen, including dose, frequency, and duration of supplementation, are outlined in Table1. A member of the field study team who was blinded to the treatment group administered the supplements and visited each child twice weekly to assess tolerance and any adverse effects of supplementation. No significant differences in the rate of adverse events were identified between the treatment groups.
Biochemical analysesBlood samples were collected from fasted subjects at 0 and 4 months, and the samples were stored at -80°C until analysis. Glutamine and arginine were measured as reported elsewhere (19). Leptin, adiponectin, IGF-1, and cortisol levels were measured in plasma samples using an enzyme-linked immunosorbent assay according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Anthropometric measurementsAnthropometric measurements were obtained at 0, 4, and 8 months. A calibrated digital weight scale with 100 g precision (Tanita Solar Scale, Tanita Corporation of America Inc., Arlington, IL) was used to weigh each child. All measurements were performed using standard methods, as described elsewhere (3). Weights were measured with a calibrated digital weighing scale with 100 g precision (Tanita Solar Scale, Tanita Corporation of American Inc., Arlington, IL). Height was measured in the supine position for children under 24 months old and standing for children aged 24 months or older using an anthropometric rod with an accuracy of 0.1 cm. As markers of physical development and nutritional status, HAZ, weight-for-age z-scores (WAZ), and weight-for-height z-scores (WHZ) were calculated using the anthropometric software Epi-Info (Centers for Disease Control, Atlanta, GA, USA). These anthropometric z-scores represent the number of standard deviations above or below the median values for the National Center for Health Statistics (NCHS) and the International Reference Population (21).
Intestinal permeability assayThe intestinal permeability assay was performed at 0 and 4 months, as described in the protocol (Figure1). The children were fasted for at least 3 hours prior to ingesting 20 mL of a solution containing lactulose (250 mg/mL; Lactulona, Luitpold Produtos Farmacêuticos Ltda., Barueri, São Paulo, SP) and mannitol (50 mg/mL; HenriFarma Produtos Químicos e Farmacêuticos Ltda., São Paulo, SP). Urine samples were collected after lactulose and mannitol ingestion and were stored at -80°C until analysis.
Sample size and statistical analysesBased on previously published data (3), a sample size of 35 for each group was chosen with an effect of 0.36 SD with 80% power (2-tailed test with a type I error of 5%) in the mean WHZ, WAZ, and HAZ changes among children receiving glutamine, glutamine plus zinc and vitamin A, or the placebo-glycine control group, with an estimate of 15% loss to follow-up. For the secondary parameter, the lactulose:mannitol ratio (pilot data: mean 0.13±0.04 SD), an estimated sample size of at least 35 subjects for each treatment group was calculated for the intervention study, assuming a 30% loss to follow-up (p<0.05, power: 80%). To assess treatment-related differences in the plasma concentrations of leptin, adiponectin, IGF-1, and cortisol, we determined an effect size of 0.7 SD with these group-wise comparisons and two-sided statistical analyses, and a sample size of approximately 41 subjects per treatment group was estimated (p<0.05, power: 80%).
The data were entered twice and were validated by three independent personnel using Access software (Microsoft Corporation, New York, NY). Epi Info Nutstat software (version 6.0; Centers for Disease Control and Prevention, Atlanta, GA) was used to calculate the nutritional parameters. Parametric (Student's t tests) and non-parametric (Mann-Whitney test) tests, along with the chi-square test or Fisher's exact test for categorical variables, were used as indicated to compare differences between the treatment groups. Either Pearson (product-moment correlation) or Spearman (two continuous variables) correlations were used to assess associations between plasma hormone levels and anthropometrics. An analysis of covariance was used to examine associations between hormone concentrations and anthropometric parameters. All statistical analyses were performed using the Statistical Package for Social Sciences, version 11.5 (SPSS Inc., Chicago, IL). P-values of 0.05 or less were accepted as statistically significant. GraphPad Prism version 3.0 (GraphPad Software, San Diego, CA) was used for figures and tables.
RESULTSThe children had an overall median age of 37 months at the study onset (range: 4.9 to 109.6 months), and the control and treatment groups were not significantly different at baseline with respect to age, sex, anthropometry, or lactulose:mannitol ratio (Table1.
The plasma concentrations of hormones at baseline were as follows (median and range): leptin = 7.0 (5.89-9.33) pg/mL; IGF = 3.57 (1.89-5.47) ng/mL; adiponectin = 9.58 (7.92-10.8) ng/mL; cortisol = 2.87 (1.34-4.19) ng/mL (Table1. Leptin and IGF-1 were positively correlated with age, height, and weight in these children at baseline (Table2. Adiponectin and cortisol decreased with increasing age, height, and weight (Table2. Only adiponectin had a significant positive correlation with HAZ. None of the intestinal barrier function parameters, such as percentage of mannitol and lactulose urinary excretion and the lactulose:mannitol ratio were significantly correlated with leptin, IGF-1, adiponectin, or cortisol (Table2. The leptin/adiponectin ratio was positively correlated with age (Spearman's correlation; n = 91; r = 0.32; p = 0.0018). Z-scores for WAZ, HAZ, and WHZ or intestinal barrier function parameters were not significantly correlated with the leptin/adiponectin ratio (Spearman's correlation; p>0.05). Only leptin had significant positive correlations with IGF-1 at baseline, and the leptin/adiponectin ratio was also positively correlated with IGF-1 and was significantly negatively correlated with cortisol (Table2.
Spearman correlation coefficients for leptin, IGF-1, adiponectin, and cortisol plasma levels vs. age, anthropometric measures, and intestinal barrier function parameters in young children.
| Parameters | Leptin | IGF-1 | Adiponectin | Cortisol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | r | p | n | r | p | n | r | p | n | r | p | |
| Age (month) | 91 | 0.205 | 0.051 | 90 | 0.457 | 0.000 | 91 | -0.246 | 0.019 | 90 | -0.223 | 0.034 |
| Height-for-age | 91 | 0.002 | 0.987 | 90 | 0.183 | 0.084 | 91 | 0.235 | 0.025 | 90 | -0.078 | 0.465 |
| Weight-for-age | 91 | 0.045 | 0.670 | 90 | 0.018 | 0.869 | 91 | 0.017 | 0.875 | 90 | -0.050 | 0.639 |
| Weight-for-height | 89 | 0.046 | 0.669 | 88 | -0.168 | 0.117 | 89 | -0.064 | 0.553 | 88 | 0.044 | 0.686 |
| % Mannitol | 89 | 0.067 | 0.532 | 88 | 0.151 | 0.160 | 89 | -0.123 | 0.251 | 88 | -0.182 | 0.089 |
| % Lactulose | 79 | 0.216 | 0.056 | 78 | 0.066 | 0.566 | 79 | 0.066 | 0.562 | 78 | -0.066 | 0.567 |
| Lact./mannitol ratio | 79 | 0.083 | 0.467 | 78 | -0.092 | 0.423 | 79 | 0.160 | 0.159 | 78 | 0.087 | 0.451 |
| Leptin | 91 | - | - | 90 | 0.522 | 0.000 | 90 | -0.014 | 0.896 | 91 | 0.137 | 0.195 |
| IGF-1 | 90 | - | - | - | - | - | 89 | -0.024 | 0.822 | 90 | 0.069 | 0.518 |
| Adiponectin | 91 | - | - | - | - | - | - | - | - | 90 | 0.55 | 0.604 |
| Leptin/Adiponectin | 91 | 0.761 | 0.000 | 90 | 0.392 | 0.000 | 90 | -0.068 | 0.522 | 91 | -0.474 | 0.000 |
n = number of children; r = Spearman correlation coefficient; p = statistical significance value.
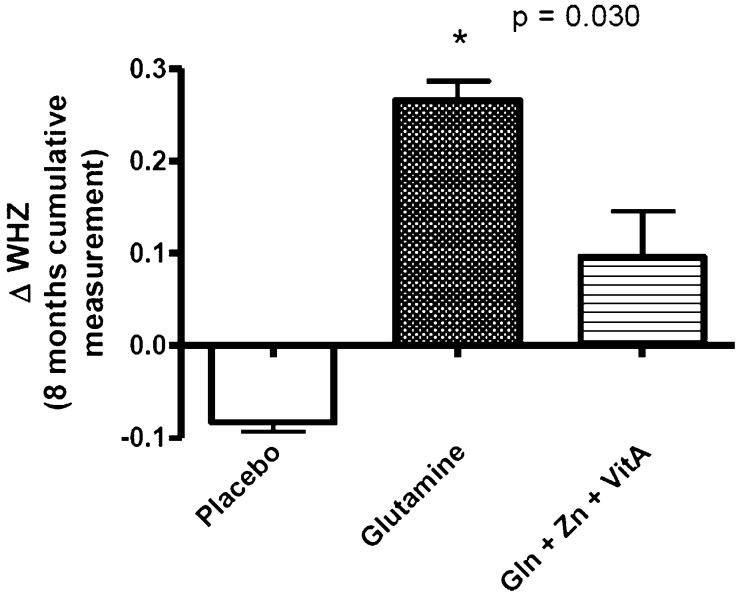
Figure2 shows the cumulative changes (final minus initial measurement for the eight-month follow-up). The cumulative changes in WHZ (adjusted for age) over the eight-month follow-up period revealed a significant increase in the glutamine group compared with the placebo group (0.26±0.02 vs. -0.08±0.01; p = 0.03); however, the changes in WAZ and HAZ were not significant (data not shown). The combination of glutamine with zinc and vitamin A improved growth; however, the improvement was not significant when compared to the placebo-glycine group (0.096±0.05; p = 0.277).
Glutamine treatment significantly reduced the percentage of lactulose (median: 0.16 and range: 0.02-3.08 vs. median: 0.52 and range: 0.01-25.17; p = 0.008) and the urinary mannitol excretion (2.99 and 0.05-24.07 vs. 6.89 and 0.17-35.5; p = 0.0038) compared with the placebo-glycine group. All of the nutrients combined also significantly decreased the lactulose:mannitol ratio compared with the glutamine-treated group (0.079 and 0.009-0.52 vs. 4.73 and 0.27-45.83; p = 0.041). The lactulose:mannitol scores were not significantly correlated with the hormone levels assessed before or after treatment with glutamine or in combination with vitamin A and zinc.
A total of 27 children (27/104; 26%) experienced adverse events, and 13 children (13/104; 12.5%) had serious adverse events. The rates of adverse and serious adverse events were similar in all study groups (p>0.05). The most common serious adverse events were respiratory infections (n = 4), pneumonia (n = 2), asthma complications (n = 6), and diarrhea (n = 1). There were no deaths.
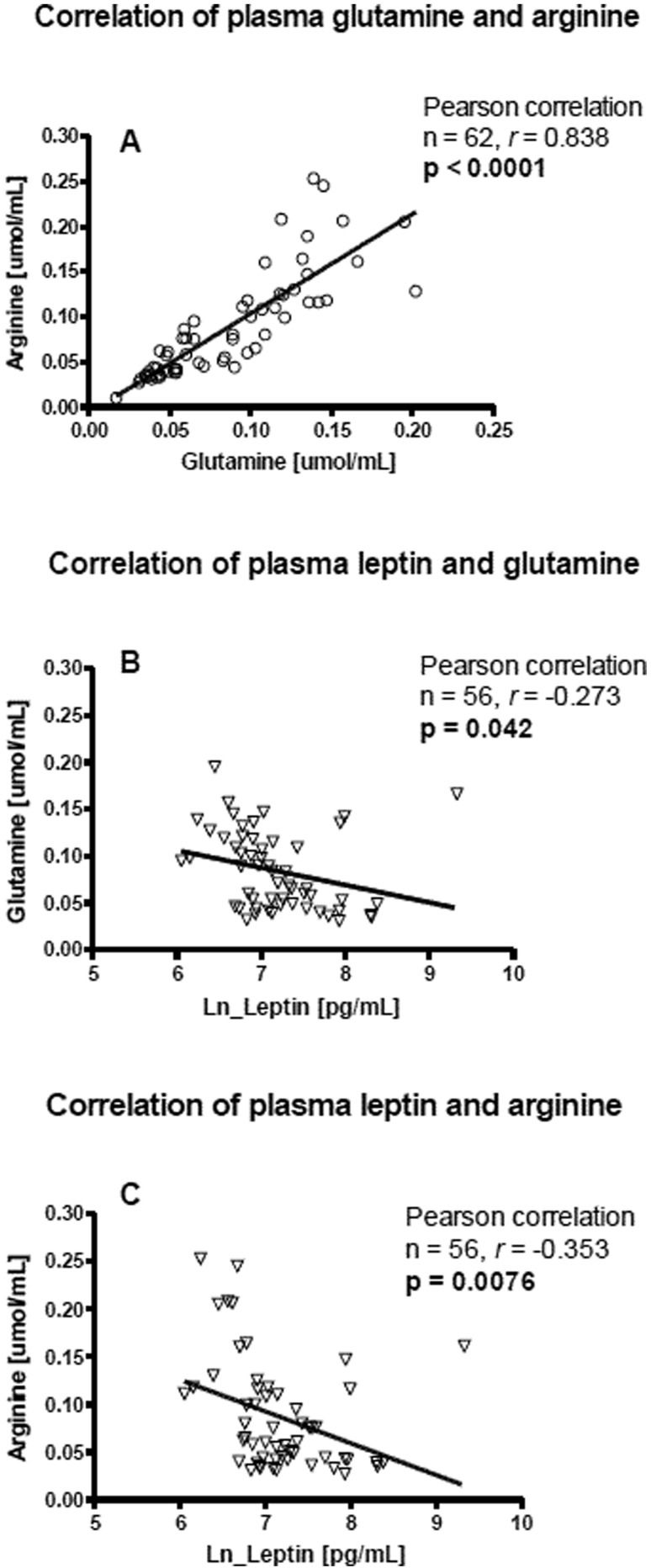
Treatment with glutamine alone or all nutrients combined did not significantly affect the selected plasma hormone levels at four months of evaluation (p<0.05). Differences identified between the treatment groups were also not altered by glutamine alone or glutamine combined with vitamin A and zinc. We examined the plasma glutamine and arginine concentrations at baseline with respect to the hormone concentrations and noted a significant positive correlation between glutamine and arginine concentrations (Pearson correlation; n = 62, r = 0.83, p<0.0001) and significant negative correlations between leptin and both glutamine and arginine (n = 56; leptin vs. glutamine, r = -0.27, p = 0.042; leptin vs. arginine, r = -0.35, p = 0.007) (Figure3). The significant negative correlations between leptin and both glutamine and arginine were abolished by 4 months of treatment with glutamine (n = 19; leptin vs. glutamine, r = -0.15, p = 0.51; leptin vs. arginine, r = -0.423, p = 0.071).
Correlation at baseline between leptin, plasma glutamine and arginine concentrations on control, glutamine alone or all nutrients combined. A. Correlation of plasma glutamine with arginine concentration. B. Correlation of leptin with glutamine concentration. C. Correlation of leptin with arginine concentrations.
Using Spearman's correlation analyses, we found that plasma leptin levels were significantly positively correlated with WHZ; however, plasma leptin levels were negatively correlated with increases in WAZ and WHZ between 0 and 4 months in the control group. Treatment with glutamine alone or in combination with vitamin A and zinc abolished the negative correlation between leptin and changes in WAZ and WHZ (delta scores), and both glutamine treatment groups exhibited significantly improved correlations between the leptin or the leptin/adiponectin ratio and WAZ/WHZ. The percentage of lactulose excretion and the lactulose:mannitol ratio were also significantly associated with leptin plasma levels in the control group at four months; however, treatment with glutamine alone or in combination with vitamin A and zinc did not show this association. Controls and subjects treated with glutamine alone or in combination with vitamin A and zinc continued to demonstrate positive correlations between IGF-1 plasma concentrations and age, height, and weight at four months. A negative correlation was observed at four months between IGF-1 and delta-HAZ, although there was a significant positive correlation between IGF-1 delta-WHZ. Treatment with glutamine alone abolished the negative correlation between adiponectin plasma concentrations and age. Because leptin levels were associated with growth parameters and leptin was associated with glutamine alone or in combination with vitamin A and zinc, we further analyzed leptin concentrations at four months (i.e., after treatment), controlling for paired initial leptin concentrations (at time 0), gender, and WAZ at time 0. Table3 summarizes these analyses. Among the subjects treated with glutamine alone or in combination with vitamin A and zinc, leptin plasma levels were significantly correlated with WAZ and WHZ, even after controlling for gender and the leptin plasma level at baseline.
Pearson correlations illustrating the relationship between plasma leptin concentrations, weight-for-age (WAZ), weight-for-height (WHZ), and change (0-4 months) in WAZ and WHZ in the placebo-glycine control and the glutamine treatment group (alone or in combination).
| Ln (Leptin) t = 4 | Ln (Leptin) t = 4 | Ln (Leptin) t = 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Variable | Controlling for ln_Leptin t = 0 | Controlling for ln_Leptin t = 0 and sex | Controlling for WAZ t = 0 | ||||||
| Time (4 months) | n | r | p | n | r | p | n | r | p | |
| Control | WAZ | 27 | 0.192 | 0.347 | 27 | 0.189 | 0.365 | 29 | 0.010 | 0.958 |
| WHZ | 27 | 0.384 | 0.053 | 27 | 0.366 | 0.072 | - | - | - | |
| Delta WAZ | 27 | -0.346 | 0.083 | 27 | -0.332 | 0.105 | 29 | -0.010 | 0.958 | |
| Delta WHZ | 27 | -0.400 | 0.043 | 27 | -0.397 | 0.050 | - | - | - | |
| Glutamine | WAZ | 25 | 0.524 | 0.009 | 25 | 0.525 | 0.010 | 30 | 0.244 | 0.202 |
| WHZ | 23 | 0.702 | 0.000 | 23 | 0.774 | 0.000 | - | - | - | |
| Delta WAZ | 25 | 0.185 | 0.387 | 25 | 0.165 | 0.453 | 30 | 0.244 | 0.202 | |
| Delta WHZ | 23 | 0.407 | 0.060 | 23 | 0.393 | 0.078 | - | - | - | |
| Glutamine plus | WAZ | 28 | 0.451 | 0.018 | 28 | 0.446 | 0.022 | 28 | 0.095 | 0.639 |
| Vitamin A and | WHZ | 27 | 0.500 | 0.009 | 27 | 0.510 | 0.009 | - | - | - |
| Zinc | Delta WAZ | 28 | 0.304 | 0.123 | 28 | 0.332 | 0.097 | 28 | 0.095 | 0.639 |
| Delta WHZ | 27 | 0.107 | 0.602 | 27 | 0.112 | 0.594 | - | - | - |
n = number of children; r = Pearson correlation coefficient; p = statistical significance value.
This double-blinded, controlled clinical trial enrolled children at risk for enteric infections and diarrhea and demonstrated that glutamine treatment significantly enhanced physical growth, as determined by improvements in cumulative WHZ, compared to the placebo-glycine group. The positive effect of glutamine on growth was consistent with our previous report showing that treatment with alanyl-glutamine (a glutamine-derivative dipeptide) improved child growth rates after a four-month follow-up compared to placebo controls in the same setting (3). Additionally, the benefit of this nutrient on the intestinal barrier function was shown by decreased lactulose epithelial permeation (i.e., reduced urinary excretion) in the lactulose:mannitol assay, which is an indicator of a more intact intestinal epithelial barrier.
Our data indicated that leptin and IGF-1 were positively correlated with age, height, and weight in these children at baseline, whereas adiponectin and cortisol plasma levels decreased with increasing age, height, and weight. Thus, these hormone levels may provide a marker not only for obesity and metabolic syndrome but also for nutritional status in children, especially during catch-up growth in children with lower HAZ (lower than the median z-score of the study population). In addition, the catch-up growth after diarrheal illnesses and the growth and satiety-related hormone levels may be strongly influenced by a genetic component (20,21).
In addition, our findings indicate that plasma leptin and the leptin/adiponectin ratio had a significant positive correlation with IGF-1; however, the leptin/adiponectin ratio had a significant negative correlation with cortisol. Leptin was originally suggested as an “anti-obesity” hormone because of its suppressive effects on appetite, which was further supported by animal studies. However, the relationships between ob/ob leptin tissue receptors and leptin signaling proved to be far more complex, as treatment of obese individuals with leptin mostly failed to promote weight loss due to increased tissue resistance to leptin (22,23).
Recently, serum leptin levels have been associated with increased body mass index in both children and adults (24). In undernourished children at baseline, plasma leptin levels have been positively correlated with IGF-1 levels and negatively correlated with cortisol levels (10,25). IGF-1, the growth hormone mediator, may function in concert with leptin to enhance childhood somatic growth (10,25). Adiponectin serum levels have been found to be consistently negatively correlated with body fat mass (26). However, in this study, adiponectin, leptin, IGF-1, and cortisol plasma concentrations were not significantly associated with body weight.
Glutamine and arginine levels in undernourished children have not been well documented. In the current study, the concentrations of these two amino acids were lower than the normal range observed in healthy children (glutamine: 0.479-0.821 μmol/mL; arginine: 0.120-0.144 μmol/mL) (3). In addition, glutamine and arginine plasma levels showed consistent and significant positive correlations with each other, suggesting that these important amino acids synergistically interact to exert beneficial effects on child growth and development. However, the mechanisms underlying these interactions remain unclear. Recently, glutamine has been associated with improved intestinal barrier function and improved child growth and development in various pre-clinical and clinical studies (3,4). Both glutamine and arginine stimulate immune responses and intestinal cell proliferation and migration through protein kinase-dependent pathways, and they appear to have an additive effect on these cells (27,28). Plasma leptin concentrations were significantly negatively correlated with plasma glutamine and arginine levels at baseline in this study. These results suggest that leptin co-regulates amino acid uptake in target cells, which might have implications for childhood growth. As shown in this work, treatment with glutamine completely abolished the negative correlation of leptin with these two plasma amino acids, suggesting that these amino acids downregulate leptin secretion and may stimulate fat deposition.
Leptin and IGF-1 showed significant positive correlations with age, and adiponectin and cortisol showed significant negative correlations with age. Previous studies have also shown that leptin and IGF-1 levels increase with age, while adiponectin and cortisol levels decrease with age (11). During puberty, leptin levels increase dramatically, whereas adiponectin levels decrease (29,30). Our findings revealed a similar pattern with respect to these hormones and weight/height. Thus, leptin and IGF-1 were significantly positively correlated with weight and height, whereas adiponectin and cortisol were significantly negatively correlated with weight and height. A recent work also reported a positive correlation of leptin and IGF-1 with body mass index and height-for-age in normally nourished children and a positive correlation of plasma IGF-1 with mid-arm circumference and height-for-age score in malnourished children (25). Researchers consistently agree that plasma leptin levels in children and adults are associated with body mass index, fat mass, insulin resistance, and dyslipidemia (24). However, adequate concentrations of leptin are necessary for immune competence because this hormone enhances thymic growth, lymphocyte proliferation, and macrophage pro-inflammatory response (31). Conversely, although adiponectin may have beneficial cardiovascular effects, excessive levels, as observed in malnutrition, may be immunosuppressive (32).
Neither glutamine alone nor glutamine given with zinc and vitamin A altered plasma concentrations of leptin, adiponectin, IGF-1, and cortisol. However, this study suggested that glutamine or all nutrients combined function in the co-regulation of leptin to improve WAZ and WHZ even after controlling for initial plasma leptin concentration and gender. Neither glutamine alone nor glutamine combined with zinc and vitamin A significantly altered the correlations of IGF-1, adiponectin, and cortisol with anthropometric measurements. One study examined the effect of zinc on leptin and ghrelin plasma concentrations and showed that this nutrient did not alter the concentrations of these hormones either during or after treatment; however, zinc improved the positive correlation between leptin and weight-for-age z-scores over a three-month period (13). Buyukgebiz and colleagues also reported that leptin was increased only in children who exhibited catch-up growth, and the increases in leptin levels were much higher in children with protein-energy malnutrition compared with healthy control children (33). The lack of an effect of these nutrients on plasma leptin concentrations does not rule out a possible influence on the leptin axis because the effect could be mediated via an excess of soluble plasma leptin receptor. Stein and colleagues showed that in severely malnourished children receiving nutritional support, leptin and the molar excess of soluble leptin receptor divided by the leptin concentration are better biomarkers of nutritional status than IGF-1 during nutritional recovery (34). Because we did not measure the soluble leptin receptor, our analysis was limited to the leptin plasma concentration. These results suggest that glutamine given alone improves child growth as measured by the change in WHZ and that this effect partially correlated with increasing plasma leptin levels. An intestinal repair effect was observed with glutamine and glutamine plus vitamin A and zinc, based on the percentage of lactulose excretion and the lactulose:mannitol ratio, respectively; however, the intestinal barrier function was not significantly correlated with these plasma hormones. Moore and colleagues also found that plasma leptin levels had no detectable relationship with the intestinal barrier function in moderately malnourished rural Gambian children (35).
The intriguing finding of a negative correlation of plasma glutamine and leptin levels at baseline may be explained by a preexisting chronic inflammatory state of the study children, that inflammatory state could have increased blood C-reactive protein levels that may have impaired the leptin binding to leptin receptors and therefore increasing its circulating levels (36). The same chronic inflammatory state may have caused a decline in plasma glutamine levels, altogether these effects would have been ameliorated with glutamine supplementation.
We acknowledge that the study population shows some age heterogeneity, which could create some bias in the study analyses, although age and seasonality were controlled for in the analysis. There were totals of thirteen, ten, and ten children below two years old in the placebo, glutamine-treated, and three-nutrient-treated (glutamine, zinc, and vitamin A) groups, respectively. The covariance analyses of growth between these groups were performed with adjustments for age and season (dry and rainy seasons in Fortaleza); thus, the possible influences of these factors were taken into consideration. We also performed independent t test analyses, and we did not identify significant differences between groups after splitting the groups into <2 years old and ≥2 years old. Another limitation was that HAZ (i.e., LAZ = length-per-age z-score for infants) might not be the best criterion for studying growth parameters in infants; however, HAZ has been indicated as a good surrogate measure of long-term growth deficits following enteric infections and diarrhea (4). To avoid the influence of any significant differences in the baseline data between groups, we used delta values (final minus initial value) for all observed growth parameters.
In conclusion, these results suggest that glutamine treatment improved growth as measured by the cumulative WHZ, possibly via interactions with leptin, as suggested by positive correlations with this satiety-related hormone. However, IGF-1, adiponectin, and cortisol were not correlated with increased WAZ and WHZ in this population. In addition, glutamine alone or all nutrients in combination (glutamine, vitamin A and zinc) improved the intestinal barrier function, which likely contributed to the growth benefits observed in these children.
ACKNOWLEDGMENTSThe Brazilian funding agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the International Collaborations in Infectious Disease Research (ICIDR) program of the US NIH (Grant # 5 U01 AI026512) supported this study.
AUTHOR CONTRIBUTIONSLima NL coordinated the surveillance team. Soares AM and Mota RM were responsible for data safety, monitoring, and statistical analyses. Anstead GM and Zhang Q conducted the data analyses. Figueiredo IL prepared the manuscript. Lima AA, Guerrant RL, and Oria RB were responsible for study design and coordination.
All authors contributed significantly to the research and have read the manuscript, which has not been published and is not under consideration for publication elsewhere.
No potential conflict of interest was reported.