To study the effects of mycophenolate sodium on mucociliary clearance.
INTRODUCTION:Mycophenolate is one of the most commonly used immunosuppressive drugs in lung transplantation. Although its pharmacokinetic properties are well defined, its side effects on mucociliary clearance have not yet been studied.
METHODS:Sixty rats were subjected to left bronchial section and anastomosis. The right bronchus was used as a control. After surgery, the rats were assigned to two groups based on whether they received saline solution (n = 30) or mycophenolate sodium (n = 30). After 7, 15, or 30 days of treatment, 10 animals from each group were sacrificed, and in vitro mucus transportability, in situ mucociliary transport velocity and ciliary beat frequency were measured.
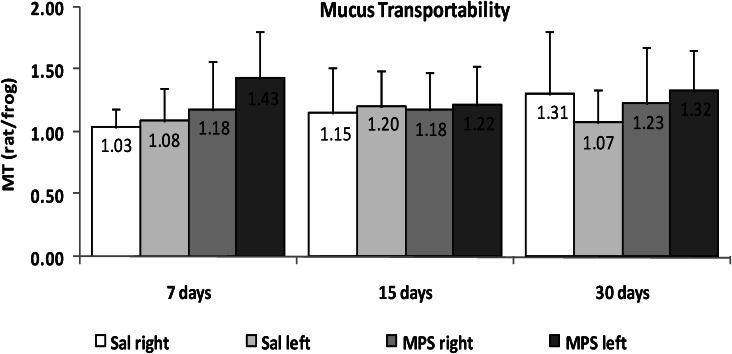
RESULTS:The analysis of mucus transportability revealed that neither mycophenolate nor bronchial section altered any transportability related property for up to 30 days of treatment after surgery (p>0.05). With regard to ciliary beat frequency, the operated left bronchi from the mycophenolate group showed a significant decrease on post-surgical day 30 (p = 0.003). In addition, we found a significant reduction in the in situ mucociliary transport velocity in the mycophenolate-treated group (p = 0.0001).
DISCUSSION:These data add important information regarding mucociliary clearance dysfunction following mycophenolate therapy and suggest that mycophenolate might contribute to the high incidence of respiratory tract infections in lung transplant patients. Further studies are needed to investigate the combined action of mycophenolate with other immunosuppressive drugs and to establish methods to protect and recover mucociliary clearance, an important airway defense mechanism.
Mycophenolate is currently one of the most commonly used immunosuppressive drugs in lung transplantation (LTx).1,2 Mycophenolate is a cell cycle inhibitor that produces reversible noncompetitive blockade of the purine synthesis pathway enzyme type II isoform of inosine monophosphate dehydrogenase.3,4 Although its pharmacokinetic properties are well defined, the side effects of mycophenolate on respiratory epithelium, and mucociliary clearance (MCC) in particular, have yet to be studied.
MCC is a first-line defense mechanism that protects the lungs from the accumulation of particles and pathogens by removing bacteria, viruses, antigens, and toxins that are trapped in the mucus on the tracheobronchial airway surface.5 An essential balance between mucus production and its removal by ciliated cells is required for optimal airway defense.6
Many studies have reported that MCC is impaired in lung transplant patients.5 Several features that are unique to LTx determine the predominance of fungal colonization and infections in the early postoperative period. Perhaps one of the most important factors is the direct communication between the graft and the outside environment. In addition, blood supply to the anastomosis is disrupted,7 causing chronic mild ischemia and placing the graft at high risk for infection.8
In previous studies, we showed that cyclosporine,9,10 azathioprine,11 and bronchial section12 can all impair MCC by decreasing ciliary activity and mucus production and secretion.
We hypothesize that mycophenolate can also play an important role in impaired MCC in a bronchial section and anastomosis rodent model. To test this hypothesis, we analyzed the components of bronchial ciliated epithelium in immunosuppressed rats by measuring ciliary beat frequency (CBF), mucociliary transport velocity (MCTV), and mucus transportability (MT).
MATERIALS AND METHODSExperimental designSixty male Wistar rats weighting 300 g were subjected to left bronchial section and anastomosis. The right bronchus was used as an internal control. Following surgery, the rats were assigned to two groups that received either saline solution (Sal group, n = 30) or mycophenolate sodium (MPS group, n = 30). The animals were maintained according to the Guide for the Care and Use of Laboratory Animals,13 and our institution's ethics committee approved the protocol.
Surgical procedureAnesthesia was induced with inhaled isoflurane (Isoforine, Cristália, Itapira, SP, Brazil) in a closed chamber, followed by orotracheal intubation with a 14-gauge catheter that was 6.5 cm in length. General anesthesia was maintained by inhalation of 2% isoflurane in pure oxygen via a nebulizer (Model 1223; Takaoka; São Paulo, SP, Brazil). Ventilation was achieved by a volume-cycled ventilator (Harvard Apparatus, Holliston, MA, USA) with a respiratory rate of 70 breaths/min and a tidal volume of 10 ml/kg. A left thoracotomy was then performed. The left main stem bronchus was dissected, clamped, and completely transected, followed by an end-to-end anastomosis with continuous running 8-0 polypropylene sutures. Finally, airflow was restored, and the atelectasis of the left lung was resolved by hyperinflation. Prior to closing the incision, a chest tube was inserted. The chest tube and tracheal tube were removed immediately after spontaneous respiration was restored. The surgical procedure was performed with the aid of a stereomicroscope at 8x the original magnification.
TherapyFollowing surgery, mycophenolate sodium (Myfortic, 180 mg, Novartis, Stein, Switzerland) was administered to the animals via an orogastric tube at a daily dosage of 400 mg/m2/day diluted in saline. The animals in the Sal group were subjected to the same therapeutic regimen but received only the vehicle (saline) in an equivalent volume.
EuthanasiaAfter 7, 15, or 30 days of treatment, ten animals from each group were anesthetized with intraperitoneal sodium pentobarbital (30 mg/kg) and sacrificed by exsanguination by section of the abdominal aorta.14
Data collectionImmediately after the animals were sacrificed, their lungs were removed en bloc from the thoracic cavity and placed in a petri dish. After dissection, an incision was made in each main bronchus, and mucus collection was performed by inserting a small hair paintbrush into the lumen of each bronchus. The mucus that adhered to the paintbrush was then placed in a 0.6-ml microtube containing mineral oil (to prevent dehydration) and stored at -70 °C. MT was measured using an in vitro frog palate model.15 The mucus, which was previously defrosted at room temperature, was placed on the frog palate ciliated epithelium, and its movement was observed and timed with the aid of a stereomicroscope equipped with a reticulated eyepiece. The MT of the rat mucus was compared to that of the frog mucus itself, and the results are therefore expressed as relative velocity (rat/frog).
After collection of the mucus sample, the bronchi were placed under a light microscope (Olympus, BX50, Tokyo, Japan) connected to a video camera (Sony Trinitron, 3CCD, Tokyo, Japan). A stroboscope (Machine Vision Strobe, Cedarhurst, NY) was placed in front of the bronchi, and CBF was measured by synchronization between cilia movement and a stroboscope flashlight.
Finally, under the same microscope, in situ MCTV was measured by direct observation of particles deposited on the mucous layer moving across the bronchi. The movement of the particles was timed, and the velocity was registered as the distance covered over one minute.
Statistical analysisAll of the data were analyzed using the Statistic Package for Social Sciences (SPSS version 13.0). An analysis of variance was used to test the interference and interaction of the factors. Comparisons between groups were performed using the Bonferroni post-hoc test. The results are expressed as mean±SD, and the differences were considered significance when p<0.05.
RESULTSIn the MT data collected from the rat mucus samples and tested in the frog palate model, we found that neither mycophenolate sodium (MPS) nor bronchial section altered any transportability property for up to 30 days of treatment after surgery (p>0.05; Figure 1).
With regard to the CBF measurements (Figure 2), the left bronchi from MPS group showed a significant decrease on postoperative day 30 (8.47±1.12 Hz) compared to day 7 (10.72±1.21 Hz, p<0.001) and day 15 (9.80±1.16 Hz, p = 0.026). In addition, a significant decrease was also observed compared to the right bronchi from MPS group at 30 days (9.73±0.98 Hz, p = 0.003).
In situ MCTV (Figure 3) was significantly slower in the MPS left group (0.021±0.009 mm/min) than in the Sal left group (0.038±0.023 mm/min, p = 0.016) after 30 days. The Sal left group showed an increase in MCTV compared to 7 (0.018±0.011 mm/min, p = 0.009) and 15 days (0.017±0.013 mm/min, p = 0.005) after the surgery and compared to the Sal right group at 30 days (0.023±0.018 mm/min, p = 0.003). After 15 days of therapy, MCTV in the MPS right group (0.038±0.017 mm/min) was higher than in the Sal right (0.018±0.016 mm/min, p = 0.014) and MPS left (0.019±0.008 mm/min, p<0.001) groups. On postoperative day 7, only the Sal left group showed MCTV impairment (0.018±0.011 mm/min) relative to the Sal right group (0.028±0.024 mm/min, p = 0.034).
Mucociliary transport velocity (MCTV) from the left (operated) or right (intact) bronchi of rats treated with saline (Sal) or mycophenolate sodium (MPS) for 7, 15, or 30 days. ∗Statistical differences between groups at each time point: 7 days - Sal right vs. Sal left, p = 0.034; 15 days - MPS right vs. Sal right and vs. MPS left, p = 0.014 and p<0.001, respectively; 30 days – Sal left vs. Sal right and MPS left, p = 0.003 and p = 0.016, respectively. Behavior of groups over time: Sal left 30 days vs. 7 and 15 days, p = 0.009 and 0.005, respectively.
In the present study, we tested a drug that is commonly used as part of the immunosuppressant triple therapy regimen that consists of a corticosteroid (prednisone, prednisolone, or methylprednisolone), a calcineurin inhibitor (cyclosporine or tacrolimus), and an antimetabolite (azathioprine or mycophenolate).1,2,16,17 We found that mycophenolate impairs MCC in the operated bronchi of animals treated for 30 days. Consistent with our previous results,10,12 MCC was also impaired by bronchial section for up to 15 days after surgery in saline-treated animals and showed significant recovery by postoperative day 30. In the immunosuppressed animals, however, this was not the case. These data partially corroborate our initial hypothesis and suggest that MPS might contribute to the high incidence of infection in the respiratory tract of lung transplant patients.
Clinicians are continually searching for an adequate immunosuppressive regimen in an attempt to maximize efficacy against rejection while avoiding toxicity and infection.17 Unfortunately, the choice of the immunosuppressive regimen for an individual patient's requirements is usually reactive rather than proactive.1 With respect to the optimal early and maintenance immunosuppression regimens, a recent review showed a strong contrast between the wealth of evidence available in the renal transplant field and the paucity, or at least a lack of consistency, of information for LTx.19 Several factors could explain this problem, including the small sample sizes and often contradictory results of original studies of LTx and patients from a heterogeneous group of pulmonary diseases who receive lung transplants, which results in widely varying patient phenotypes and individual pharmacogenomics.16
Based on these difficulties, the advantages of conducting studies using animal models are clear. We believe that our bronchial section rodent model is useful for clarifying the adverse effects of immunosuppressive drugs on the respiratory epithelium, specifically on mucociliary clearance. We previously demonstrated that cyclosporine impaired both ciliary beat frequency and mucus production/secretion for up to 90 days of treatment.9,10 Conversely, azathioprine caused a transiently perturbed on mucociliary transport on postoperative days 7 and 15, and function was fully recovered in the animals that were treated for 30 days.11 Together, these results reflect the controversy we find in the literature regarding immunosuppression therapy for LTx, even regarding drugs of the same class such as azathioprine and mycophenolate, both of which are cell cycle inhibitors.
The use of mycophenolate (in place of azathioprine) has increased since its commercialization and testing in renal and heart transplantation in the early 1990s. Some studies suggest that mycophenolate may be more efficient than azathioprine at preventing acute rejection in LTx, whereas the single prospective, randomized study that compared these two drugs did not find any benefit.18 Korom et al.2 concluded in their review that there is currently no unambiguous evidence to support the superiority of mycophenolate over azathioprine in LTx that could justify its wide-scale use as a first-choice antimetabolite.
Immunosuppression by mycophenolate occurs by virtue of its active component mycophenolic acid (MPA) through the inhibition of inosine monophosphate dehydrogenase (IMPDH). T and B cells are more dependent on this pathway than are other cells. Other potential mechanisms of immunosuppression include inducing apoptosis in activated T cells, decreasing the expression of adhesion molecules (thereby decreasing the recruitment of inflammatory cells), and decreasing the production of inducible nitric oxide and the resulting tissue damage.16 Mycophenolate has also been shown to inhibit the proliferation of smooth muscle cells, fibroblasts, and endothelial cells by blocking DNA synthesis in the S phase of the cell cycle.20
To our knowledge, this is the first study to directly test the effects of mycophenolate on the mucociliary apparatus. Furthermore, we separately analyzed each of the two principal components of this clearance mechanism, thereby showing that only cilia movement from the left (sectioned) bronchus was impaired after MPS therapy for 30 days. By itself, this treatment did not alter the viscoelastic properties of the mucus, at least in the frog palate model employed here.
There are several techniques for monitoring MCC, the most common of which is the use of insoluble particles that can be directly observed, primarily in humans, through a bronchoscope, by radiography or by external monitoring of radiolabelled particles.21 In our rodent model, we used a microscope equipped with a reticulated eyepiece, which permitted the direct observation and timing of the progression of the particles trapped in the mucus layer across the bronchus. Moreover, we developed a setup consisting of an optic fiber connected to a stroboscope for analyzing CBF.10 A limiting point in this setup is its dependence on an expert researcher to analyze and register CBF according to the frequency of the stroboscopic flashlight. Finally, we used the bullfrog palate, a well-established method for analyzing the transportability of mucus samples.15 Because of its similarity with mammalian respiratory epithelium, we could test the transportability properties of the rat mucus samples in comparison with the autologous (frog) mucus. We have thus accumulated a considerable amount of information regarding the physiological variability of mucociliary clearance as a result of the effects of various immunosuppressant agents.9–11
Presently, we do not intend to analyze the intracellular mechanisms of action of MPS in the cells of the mucociliary system. We can, however, infer from our results that MPS affects ciliated cells more than secretory (goblet) cells. This is important for future studies that aim to avoid this undesirable side-effect of mycophenolate therapy on MCC. Presumably, the most informative method to study the mechanism of MPS is to use cultured respiratory epithelial cells. Given that we observed a reduction in CBF, we propose including a well-known ciliary stimulant such as extracellular adenosine triphosphate or acetylcholine22 when testing the effects of MPS.
This study was performed at the Thoracic and Cardiovascular Surgery Post-Graduation Program of Heart Institute of Clinics Hospital of São Paulo University Medical School, São Paulo, SP, Brazil. The study was supported by grants from The State of São Paulo Foundation (FAPESP), São Paulo, SP, Brazil.