Although it is known that obesity, diabetes, and Kawasaki's disease play important roles in systemic inflammation and in the development of both endothelial dysfunction and cardiomyopathy, there is a lack of data regarding the endothelial function of pre-pubertal children suffering from cardiomyopathy. In this study, we performed a systematic review of the literature on pre-pubertal children at risk of developing cardiomyopathy to assess the endothelial function of pre-pubertal children at risk of developing cardiomyopathy. We searched the published literature indexed in PubMed, Bireme and SciELO using the keywords 'endothelial’, 'children’, 'pediatric’ and 'infant’ and then compiled a systematic review. The end points were age, the pubertal stage, sex differences, the method used for the endothelial evaluation and the endothelial values themselves. No studies on children with cardiomyopathy were found. Only 11 papers were selected for our complete analysis, where these included reports on the flow-mediated percentage dilatation, the values of which were 9.80±1.80, 5.90±1.29, 4.50±0.70, and 7.10±1.27 for healthy, obese, diabetic and pre-pubertal children with Kawasaki's disease, respectively. There was no significant difference in the dilatation, independent of the endothelium, either among the groups or between the genders for both of the measurements in children; similar results have been found in adolescents and adults. The endothelial function in cardiomyopathic children remains unclear because of the lack of data; nevertheless, the known dysfunctions in children with obesity, type 1 diabetes and Kawasaki's disease may influence the severity of the cardiovascular symptoms, the prognosis, and the mortality rate. The results of this study encourage future research into the consequences of endothelial dysfunction in pre-pubertal children.
There are similarities between children and adults suffering from heart failure (HF), such as the preferred pharmacological treatment (1), the use of pace-makers and heart transplants (2,3), the inability of the patient to reach the predicted heart rate for the patient's age during cardiopulmonary exercise testing (4,5), and the ergoespirometric response under similar clinical conditions (5). In adults, endothelial dysfunction is related to the development of diastolic dysfunction (6,7), Chagas disease, left ventricular hypertrophy (8), ischemic cardiomyopathy, HF (8,9), obesity, type 1 diabetes, hyperlipidemia, arterial hypertension (10), peripheral arterial disease, chronic kidney disease (11) and atherosclerosis (12) because the dysfunction predisposes the vasculature to vasoconstriction, leukocyte adherence, platelet activation, and vascular inflammation (13). Nevertheless, there is a lack of data regarding endothelial function in children with cardiomyopathy.
The severity of endothelial dysfunction is related to the cardiovascular risk (14), the severity of cardiovascular symptoms (15), and the inability to exercise (11) and represents a predictor for cardiac transplant and death (16).
It is known that diseases, such as Kawasaki's disease (8), hyperlipidemia (10), obesity, and type 1 diabetes, play important roles in systemic inflammation and endothelial dysfunction (17). These diseases may increase the likelihood of cardiovascular events (18) and may predispose children to the development of cardiomyopathy. Based on these considerations, we reviewed the published literature on endothelial function in pre-pubertal children to evaluate the endothelial function in pre-pubertal children with cardiomyopathy or children at risk of developing cardiomyopathy, and we conducted an analysis of the data from the relevant studies. This analysis was undertaken to help clarify the role of endothelial impairment in children at risk of suffering from cardiomyopathy.
Endothelial function can be analyzed by noninvasive methods, including ultrasonography (US) (19) and peripheral artery tonometry (PAT) (20). During a US examination, the baseline rest image of the subject's brachial artery is acquired, and a 5-min arterial occlusion is performed using cuff inflation to at least 50 mm Hg suprasystolic pressure. The subsequent cuff deflation induces reactive hyperemia that results in an increase in flow or, more precisely, shear stress by dilating the brachial artery; this phenomenon is designated flow-mediated dilatation (FMD). After returning to the baseline, a second brachial artery image is recorded after the administration of nitroglycerine (NTG); this image corresponds to the contribution of the intima muscle relaxation to the dilation and is known as the endothelium-independent vasodilatation (19).
In contrast to US, the PAT evaluation is a method that does not require the administration of drugs, and it combines the assessment of the flow-mediated dilatation after the same 5-min arterial cuff occlusion, with the arterial pulse wave amplitude measurement taken using a pneumatic fingertip probe (20).
Literature search strategyA search of the PubMed, Bireme, and SciELO databases was conducted to perform a systematic review, according to the recommendations of PRISMA (21). The search was performed using the following keywords: endothelial, child, pediatrics, and infant. The results were limited to human studies published in English, Spanish, and Portuguese. The initial selection was based on the title and abstract, and those deemed relevant were retained for further analysis.
The exclusion criteria included the following: analyses of the endothelial function in animals, cadavers, adolescents or adults; reviews; analyses of either the coronary or pulmonary arteries or the neurological or osteomuscular systems; if the patients had rheumatic, oncohematological, splenic or hepatic diseases; or if the studies were trials related to markers, genetics and interventions.
The list of potential trials to include was verified by the authors of the present study. We note that there may be a risk of bias across the studies because not all of the trials published have been indexed in the selected databases.
Collected dataThe following data were collected for analysis: the number of patients enrolled in the study; the patient's age; whether sexual maturity had been reached; any diseases involved; the protocol used for the endothelial function evaluation and the FMD, NTG, and PAT values. Collected data in all tables were expressed following disease order, in order to better clarify data presentation and analysis.
Statistical analysisThe values of endothelial function were expressed as the mean ± standard deviation (SD), as collected from the original trials. The data from healthy children were compared with the data for unhealthy children, and the differences were analyzed using the Student t-test. A p-value <0.05 was considered statistically significant.
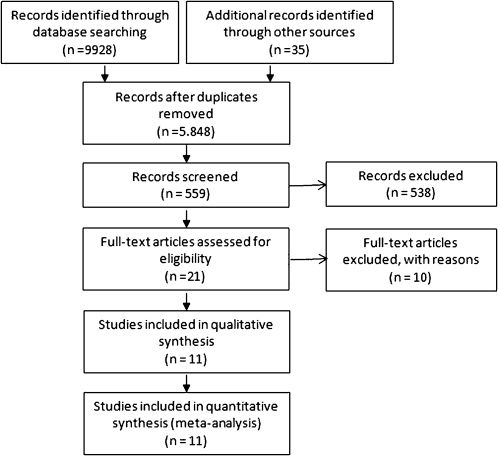
A total of 559 articles were retrieved after the preliminary search, and 21 articles were considered potentially relevant based on the title and abstract. A careful analysis of these articles was performed, and ten trials were excluded for several reasons. Eleven articles were chosen for the final analysis (Figure 1).
All of the selected studies were dated from 1996 to 2009. Table 1 presents the general descriptions of the included studies and provides information regarding the participants, their ages, their diseases, the endothelial evaluation method used and whether there were differences between genders (Table 1).
Study characteristics of the trials included in this review.
| Study | Year of publication | N | Age (years) | PS | Disease | Method | Difference in gender |
|---|---|---|---|---|---|---|---|
| Germain et al. (19) | 2004 | 32 | 9.9 | no | none | US | no |
| Aggoun et al. (20) | 2002 | 130 | 12.0 | yes | obesity | US | NI |
| Kapiotis et al. (21) | 2006 | 92 | 12.0 | no | obesity | US | no |
| Aggoun et al. (22) | 2008 | 71 | 8.8 | yes | obesity | US | no |
| Woo et al. (23) | 2004 | 73 | 10.3 | yes | obesity | US | NI |
| Pena et al. (24) | 2006 | 270 | 13.7 | no | obesity, diabetes | US | NI |
| Järvisalo et al. (25) | 2004 | 75 | 11.0 | no | diabetes | US | no |
| Wiltshire et al. (26) | 2006 | 55 | 13.7 | yes | diabetes | US | NI |
| Haller et al. (27) | 2007 | 64 | 14.6 | no | diabetes | PAT | NI |
| Borzutzky et al. (28) | 2008 | 22 | 10.2 | no | Kawasaki | US | no |
| Deng et al. (29) | 2002 | 56 | 7.1 | no | Kawasaki | US | NI |
PS, puberty state evaluated; Method, evaluation method of endothelial function; US, ultrasound; PAT, peripheral artery tonometry; NI, no data.
All eleven of the selected studies provided data for healthy children for comparison, and, with the exception of one study (22), all of the studies compared healthy children to ill children. Four of the studies evaluated obese children (23–26), one evaluated children with obesity and type 1 diabetes (27), three studied children with type 1 diabetes (28–30), and two evaluated children with Kawasaki's disease (31–32). No trial involving children with cardiomyopathy was found.
These reviewed studies reported a mean age of 9.8±1.8 years old, and only four articles investigated the sexual maturation of the group of pre-pubertal children (23,25,26,29). Forty-five percent of all of the studies found no difference in the endothelial function between genders, and 55% of the reports did not provide these data (Table 1).
Most of the studies used ultrasound to determine the percent diameter changes for the FMD and NTG assessments (22–29,31,32). Only one study used reactive hyperemia–peripheral artery tonometry (RH-PAT) for the same purpose (30), showing that the endothelial function was impaired in diabetic children relative to that in healthy children (1.63±0.5 vs. 1.95±0.3 for diabetic children vs. healthy children) (30).
The FMD response values from all of the other articles for obese and diabetic children were significantly lower than those for healthy children (5.9±1.29% for obese children, 4.5±0.7% for diabetic children, and 9.8±1.8% for healthy children; p<0.0008). The obese children had a 39% lower FMD response than the healthy children, and the diabetic children had a decrease in their FMD of 25% (Table 2).
The children with Kawasaki's disease had 27% lower FMD responses than the healthy children (7.1±1.27% vs. 9.8±1.8%, respectively), but this difference was not statistically significant (p = 0.26). The FMD data for the obese children, diabetic children, and children with Kawasaki's disease exhibited no statistically significant differences (p<0.38) (Table 2).
Percentage of endothelium-dependent vasodilatation (flow-mediated dilatation [FMD]) and percentage of endothelium-independent vasodilatation after drug administration (NTG).
| Study | Mean FMD (%) in children | Mean NTG (%) in children | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| healthy | obese | diabetic | Kawasaki's disease | p-value | healthy | obese | diabetic | Kawasaki's disease | p-value | |
| Germain et al. (19) | 10 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Aggoun et al. (20) | 8 | 6 | NA | NA | 0.010 | 18 | 17 | NA | NA | >0.05 |
| Kapiotis et al. (21) | 11 | 7.7 | NA | NA | 0.006 | NA | NA | NA | NA | NA |
| Aggoun et al. (22) | 8.3 | 4.5 | NA | NA | 0.001 | 25.8 | 19 | NA | NA | >0.05 |
| Woo et al. (23) | 9.7 | 6.6 | NA | NA | 0.001 | 19.6 | 20.6 | NA | NA | >0.05 |
| Pena et al. (24) | 7.8 | 4.9 | 3.8 | NA | 0.001 | 27.7 | 21.7 | 20.5 | NA | 0.01 |
| Järvisalo et al. (25) | 8.7 | NA | 4.4 | NA | 0.001 | 11.5 | NA | 9.7 | NA | >0.05 |
| Wiltshire et al. (26) | 9.1 | NA | 5.2 | NA | 0.002 | 23.3 | NA | 19.5 | NA | >0.05 |
| Borzutzky et al. (28) | 11.1 | NA | NA | 8.0 | >0.050 | NA | NA | NA | NA | NA |
| Deng et al. (29) | 14.1 | NA | NA | 6.2 | <0.001 | 33.2 | NA | NA | 30.6 | >0.05 |
| Mean±SD | 9.8±1.8 | 5.9±1.29 | 4.5±0.7 | 7.1±1.27 | 0.001∗ | 22.7±7.1 | 19.6±2.0 | 16.6±5.9 | - | >0.05† |
p-value, data significance; NA, not applicable; NS, not significant; ∗p<0.0005, healthy vs. obese children; p<0.0008, healthy vs. diabetic children; †p<0.11, healthy vs. obese children; p<0.19, healthy vs. diabetic children.
The NTG response (Table 2) also showed no significant difference among the groups (22.7±7.1% for healthy children, 19.6±2.0% for obese children and 16.6±5.9% for diabetic children; p<0.19).
Among the excluded studies, one contained no data on endothelial function (33), and most of the excluded studies did not list the data for pre-pubertal children, post-pubertal children (34–39) and adults (40,41) separately. Another study (42) published data for the same patients as used in a previously published study (32).
Ultrasound is the most frequently used method to evaluate endothelial function because it has a low cost, is safe and is sufficiently reproducible. However, some limitations of this method are that the FMD response can be influenced by both temperature and age, and ultrasound requires drug administration and the recording of images for posterior analysis (19).
Another technique for the assessment of endothelial function is the PAT method, which has been proposed to be more practical and precise (20), and PAT results are moderately significantly correlated with US results (43). The PAT method combines the traditional flow-mediated dilatation measurement with pneumatic fingertip probes to measure the arterial pulse wave amplitude (20). One study (31) chose PAT over US and confirmed that diabetic children have endothelial dysfunction, as do adults (44,45).
In five studies (22,24,25,28,32), the gender of the individual did not correlate with the endothelial function, as has been found for adults (60) and adolescents (61). Pre-pubertal children show lower responses to beta-adrenergic receptors and have lower levels of circulating catecholamines (adrenaline and noradrenaline) than adolescents and adults (46). The obese children exhibit accelerated growth and, therefore, earlier puberty (47), which is evidenced by an advancement of menarche by 2.2 years (11.2 vs. 13.4 years, respectively) (48) because fat mass influences the levels of hormones (49,50).
These data reinforce the need for the evaluation of sexual maturation in studies involving children. The extent of sexual maturation is widely accessed using the Tanner-Whitehouse scale, which is based on secondary sexual characteristics, such as breast development and menarche in girls, standards for penis development in boys, and pubic hair development in both sexes (51). This lack of sexual maturity assessment explains why those trials that combined information for children, adolescents (33–39), and adults (40,41) were excluded in the present study and explains the elevated FMD data from one study (24), which included a higher mean age of obese children of 12±4 years old. The evaluation of sexual maturity also explains why three of the included studies (23,25,26) that assessed the pubertal stage by clinical examination yielded similar FMD data.
Diabetic and obese individuals have hyperinsulinemia, and both diabetes and obesity are related to the higher hormone (50) and inflammation levels (52) that contribute to increased arterial stiffness and vascular lesions, which are related to both endothelial function and structural arterial changes (53) and may explain the endothelial dysfunction results for these groups (23–30).
In addition to some methodological disparities, all of the studies showed significantly impaired endothelial function (FMD response) in the obese and diabetic children despite the lack of a difference in the NTG response, a result that was reported for adults (54) when compared with healthy subjects (55). However, FMD impairment seems to be greater in adults than in children (44% in obese adults and 55% in diabetic adults); similarly, adults show no difference in NTG responses (54,56).
The collected FMD values in children with Kawasaki's disease are lower than those in healthy children (31,32), which can indicate a low, but persistent, level of inflammation (57), as evidenced by higher C-reactive protein (CRP) levels (52,53,58). Only one study (32) included NTG data, and this lack of data limits the discussion of the information provided.
The FMD values suggest that the major dysfunctions in these children occur as a result of the local nitric oxide (NO) bioavailability in the endothelium (NO production) because the shear stress induced by reactive hyperemia activates endothelial NO production (59).
The NTG response also suggests that the vascular smooth muscle cell function is preserved (19). Yet, one study showed significantly lower GT values in obese children than in healthy children (27), and two other studies did not publish values for the NTG response (22,24). Some reports (20,25,28) did not describe how long the subjects were at rest, according to Corretti et al. (22), >10 min is recommended, between the collections of the FMD and NTG response images, which might have contributed to the reported outcomes. It is also worth noting that three of the trials (24,27,29) allowed the arteries to return to the basal condition and that two of these studies (24,29) did not yield statistically significant data.
LimitationsIn spite of the statistically significant differences in some of the endothelial function responses, the number of trials regarding this subject remains limited. Another limitation of the data search for pre-pubertal children is that the studies list the results for adults, teenagers, and children together, and do not divide the data by group according to the puberty state and age. The small number of studies in children may also be due to the use of the US method because drug administration is required. However, the new method of endothelial function evaluation, the PAT method, might help increase the number of trials using this population.
Although endothelial dysfunction has been identified in adults as a predictor of cardiac transplant and death, the clinical implications of endothelial dysfunction were not presented in the selected trials, nor were they the focus of the current study; however, this correlation should be further evaluated in children. Lastly, even though the number of pre-pubertal children with endothelial dysfunction is unknown, there is a great number of children who suffer from this condition. Thus, the barriers in the pediatric field must be broken, and more studies on this topic should be performed to understand this population better.
In conclusion, endothelial function is an important clinical feature because it may indicate the severity of cardiovascular symptoms, prognosis, and mortality. Children at risk of developing cardiomyopathy exhibit endothelial dysfunction; however, the prevalence of endothelial dysfunction in cardiomyopathic children remains unknown because of the lack of data. We suggest that attention should be paid to the consequences of endothelial function in this group.
AUTHOR CONTRIBUTIONSTavares AC organized the trials' selection and the records in the tables, performed the analysis, and wrote the manuscript. Guimaraes GV was responsible for the literature search and provided assistance to the literature revision and manuscript writing. Bocchi EA provided assistance to the manuscript writing.
Guilherme V Guimarães (CNPq # 304733/2008-3) was supported by Conselho Nacional de Pesquisa.
No potential conflict of interest was reported.