Oxidative stress plays an important role in skeletal muscle damage in sepsis. Aerobic exercise can decrease oxidative stress and enhance antioxidant defenses. Therefore, it was hypothesized that aerobic exercise training before a sepsis stimulus could attenuate skeletal muscle damage by modulating oxidative stress. Thus, the aim of this study was to evaluate the effects of aerobic physical preconditioning on the different mechanisms that are involved in sepsis-induced myopathy.
METHODS:Male Wistar rats were randomly assigned to either the untrained or trained group. The exercise training protocol consisted of an eight-week treadmill program. After the training protocol, the animals from both groups were randomly assigned to either a sham group or a cecal ligation and perforation surgery group. Thus, the groups were as follows: sham, cecal ligation and perforation, sham trained, and cecal ligation and perforation trained. Five days after surgery, the animals were euthanized and their soleus and plantaris muscles were harvested. Fiber cross-sectional area, creatine kinase, thiobarbituric acid reactive species, carbonyl, catalase and superoxide dismutase activities were measured.
RESULTS:The fiber cross-sectional area was smaller, and the creatine kinase, thiobarbituric acid reactive species and carbonyl levels were higher in both muscles in the cecal ligation and perforation group than in the sham and cecal ligation and perforation trained groups. The muscle superoxide dismutase activity was higher in the cecal ligation and perforation trained group than in the sham and cecal ligation and perforation groups. The muscle catalase activity was lower in the cecal ligation and perforation group than in the sham group.
CONCLUSION:In summary, aerobic physical preconditioning prevents atrophy, lipid peroxidation and protein oxidation and improves superoxide dismutase activity in the skeletal muscles of septic rats.
Severe sepsis has been associated with an uncontrolled systemic inflammatory response to infection that is associated with developing organ dysfunction (1). Among these dysfunctions, severe myopathy plays an important role in prolonged intensive care unit stays and late sequelae (2-6).
The main signs of sepsis-induced myopathy are generalized weakness, fatigue, atrophy (7) and a delay in weaning from mechanical ventilation (8). These symptoms are associated with a pronounced catabolic response in skeletal muscle that results from proteolytic stimulation and inhibited protein synthesis (9,10) (particularly in the myofibrillar proteins) and can negatively impact patient recovery (11).
Oxidative stress plays an important role in skeletal muscle dysfunction in different clinical settings, including sepsis (12,13). Reactive oxygen species (ROS) production is exacerbated in sepsis disease mainly because of mitochondrial dysfunction (13) which in turn promotes an imbalance in the redox status because the antioxidant defenses do not increase in the same proportion. This altered redox balance causes cell damage, particularly in proteins, lipids and DNA (14).
Because aerobic exercise training induces higher oxygen consumption in parallel with increased ROS production (15) an antioxidant system is launched during such training to maintain an adequate redox balance (16). In addition, exercise training improves mitochondrial function and even increases the number of muscle mitochondria (17,18). The net effects of training are improved efficiency of the antioxidant defenses and oxygen consumption, which could be useful for scavenging ROS in sepsis (16). Therefore, aerobic exercise training enhances the antioxidant defenses in various tissues, particularly in the skeletal and cardiac muscles (19).
As an ROS scavenger strategy, the impact of exercise training on sepsis-induced muscle damage has never been investigated (to the best of our knowledge). Therefore, it was hypothesized that aerobic exercise training before the presentation of a sepsis stimulus could attenuate skeletal muscle damage by modulating oxidative stress. Thus, the aim of this study was to evaluate the effects of aerobic physical preconditioning on the different mechanisms that are involved in sepsis-induced myopathy.
MATERIALS AND METHODSAnimalsAdult male Wistar rats (70 days old) were obtained from the UNIVALI (Universidade do Vale de Itajai, Itajai, Brazil) breeding colony. The rats were maintained in a 12/12-hr light-dark cycle in a temperature-controlled (22°C) environment with free access to standard laboratory chow (protein 20 kcal%, carbohydrates 70 kcal%, and lipids 10 kcal%, Nuvital Nutrientes, Curitiba, Paraná, Brazil) and tap water. Initially, the animals were randomly assigned to the untrained and trained groups. After completing the eight-week aerobic exercise training protocol, animals in the trained group were randomly assigned to either the sham or CLP (cecal ligation and perforation) surgery group. The untrained group was subjected to the same surgical procedures. At this phase, there were four groups: 1) sham trained (ShamT), 2) CLP trained (CLPT), 3) sham and 4) CLP. All of the rats were euthanized five days post-surgery. This study was conducted in accordance with the ethical principles in animal research adopted by the Brazilian College of Animal Experimentation (www.cobea.org.br) and was approved by the UNIVILLE (Universidade da Região de Joinville, Joinville, Brazil) Ethics Committee (Protocol No. 008/08 – COEA).
Graded treadmill exercise testBefore the first exercise test, the rats were conditioned to exercise on a treadmill over a week-long period (10 min of exercise per session). During the graded treadmill exercise test, the rats were placed on a treadmill and allowed to acclimatize for at least 15 min. The exercise intensity was then increased by 3 m/min (6-33 m/min) every three minutes at a 0% grade until exhaustion (the point of maximum running speed). The graded treadmill exercise test was performed prior to the exercise training and then during the fourth and eighth weeks of exercise training. Exercise capacity was estimated by the total distance run and correlated with the skeletal muscle capacity, which is a method used to detect exercise intolerance. Thus, exercise capacity was evaluated using a graded treadmill exercise protocol, as previously described (20).
Aerobic exercise training protocolThe aerobic exercise training protocol consisted of an eight-week program of running on a motorized treadmill (KT-4000 model INBRAMED, RS, Brazil), five days a week, for 60 minutes per sessions at 60% of the maximum running speed that was obtained in the graded treadmill test, which corresponded to the maximal lactate steady-state workload (MLSSw), as described elsewhere (20). All of the untrained rats were exposed to treadmill exercises (5 minutes) three times per week to become accustomed to the exercise protocol and handling.
Cecal ligation and perforation surgeryThe animals in both the untrained and trained groups were subjected to the surgical procedure 72 hours after the last treadmill exercise test, as previously described (21,22). For the CLP surgery, the rats were anesthetized with ketamine (80 mg/kg), which was administered intraperitoneally. Under aseptic conditions, a 3-cm midline laparotomy was performed to expose the cecum with the adjoining intestine. The cecum was tightly ligated with a 3.0-silk suture at its base (below the ileocecal valve) and was perforated once with a 14-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces from the perforation site before being returned to the peritoneal cavity. The laparotomy was then closed with 3.0 silk sutures. All of the animals received isotonic saline solution (30 ml/kg subcutaneously) immediately after the CLP surgery, as well as antibiotics (ceftriaxone 30 mg/kg and clindamycin 25 mg/kg) starting 6 hr after the CLP surgery and again every 6 hr up to 24 hr after the CLP surgery. Afterward, the animals were returned to their cages with free access to food and water. In the sham group, the rats were submitted to all of the surgical procedures and received isotonic saline solution immediately after the surgery. They also received the same antibiotics as the CLP groups, but their ceca were not ligated or perforated.
To minimize the possibility that the animals did not truly develop sepsis, the CLP procedure was always performed by the same investigator. In addition, the animals were observed after the CLP surgeries for signs of infection, such as piloerection, lethargy, tachypnea, and weight loss (21,22). The animals were weighed daily for five days after the surgery, and their soleus and plantaris muscles were harvested at the time of euthanization for later analysis. The rate of sepsis survival in this model was approximately 40%.
Fiber CSAThe fiber cross-sectional area (CSA) in the soleus and plantaris muscles was used as an indicator of muscle atrophy. Liquid nitrogen-frozen muscles were vertically mounted and serially sectioned. The muscle sections were then incubated in alkali (mATPase, pH 10.3) or acid (mATPase, pH 4.6), as described by Brooke & Kaiser (23) to assess myofibrillar ATPase activity. The myosin ATPase reaction was used to identify the muscle fiber type (Figure 1). Fiber CSA was evaluated in five areas at 200× magnification and further analyzed using a digitizing unit connected to a computer (Image-Pro Plus; Media Cybernetics, Silver Spring, MD). All of the analyses were conducted by a single observer (CWC) who was blinded to the rat group assignments.
CK activityThe creatine kinase (CK) activity was measured in the tissue homogenate of the soleus and plantaris muscles. The reaction mixture for the creatine kinase assay contained 100 mM Tris-HCl buffer (pH 7.5), 30 mM phosphocreatine, 20 mM glucose, 12 mM magnesium acetate, 10 mM diadenosine pentaphosphate, 15 mM sodium azide, 20 mM n-acetylcysteine, 2 mM ADP, 5 mM AMP, 2 mM NADP, 3500 U/L hexokinase, 2000 U/L glucose-6-phosphate dehydrogenase and approximately 1.5 mg of protein in a final volume of 1200 mL. The creatine kinase activity was calculated based on reduced nicotinamide adenine dinucleotide (NADH) formation, which was monitored with a spectrophotometer at a wavelength of 340 nm at 37°C (24).
TBARSLipid peroxidation in the soleus and plantaris muscles was measured by the formation of thiobarbituric acid reactive species (TBARS) during an acid-heating reaction, as previously described (25). Briefly, the samples (200 µl) were mixed with trichloroacetic acid (10%) (400 µl) and centrifuged for 10 minutes (4000×g), and the supernatant was mixed with an equal volume of 0.67% thiobarbituric acid (TBA). The mixture was then heated in a boiling water bath for 15 minutes. The formation of TBARS was determined by measuring the absorbance at 535 nm.
Protein carbonylsThe oxidative damage to the protein in the soleus and plantaris muscles was measured by determining levels of the carbonyl groups based on the reaction of the groups with dinitrophenylhydrazine (DNPH) (26). The proteins were precipitated by adding 20% trichloroacetic acid and reacted with DNPH. The samples were then re-dissolved in 6 M guanidine hydrochloride, and the carbonyl contents were determined by measuring the absorbance at 370 nm.
CAT and SOD activityTo determine catalase (CAT) activity, the soleus and plantaris muscles were sonicated in a 50-mM phosphate buffer, and the resulting suspension was centrifuged at 3000×g for 10 minutes. The supernatant was used for the enzymatic assay. CAT activity was measured by the rate of decrease in hydrogen peroxide absorbency at 240 nm (27). Superoxide dismutase (SOD) activity was assayed by measuring the inhibition of adrenaline auto-oxidation, as previously described (28).
Statistical analysisThe data are presented as the means and standard errors of the means (mean±SEM). A two-way ANOVA with Fisher’s post hoc test (Statistica software, StatSoft, Tulsa, OK) was used to compare the effects of training and surgery in all of the analyses except for the analyses of body weight and running distance, for which a repeated-measures two-way ANOVA with Fisher’s post hoc test and Student’s t-test, respectively, were used. Statistical significance was set at p<0.05.
RESULTSIn the trained group, there was a significant increase in the running distance (375.3±37.9 m) compared with the untrained group (175.3±13.3 m) (p<0.05).
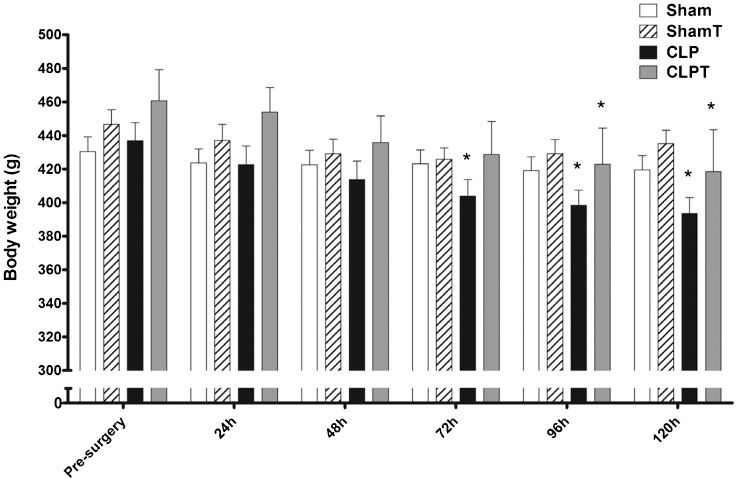
The pre-training weights of the trained (333.9±5.8 g) and untrained (332.8±5.8 g) groups were similar. After eight weeks of aerobic exercise training, during the pre-surgery time interval, the trained groups (ShamT and CLPT) exhibited an 18.7-g increase in body weight compared with the untrained groups (Sham and CLP), but this increase was not statistically significant (p = 0.11). Seventy-two hours after surgery, the CLP group exhibited a significant decrease in body weight compared with their weight during the pre-surgery period, and the CLPT group exhibited a similar decrease after 96 hours (Figure 2).
The CSA of muscle fibers was smaller in the CLP group than in the other groups. Myosin ATPase staining of the skeletal muscle sections showed that in the soleus, only the type I fibers from the CLP group were smaller than those from the other groups (p<0.05). In the plantaris muscle, only the type II fibers were smaller in the CLP group than in the other groups (p<0.05) (Figure 3).
Cross-sectional area (CSA) of type I and II fibers in the soleus (A and B) and plantaris (C and D) muscles at five days after surgery. The animals were divided into four groups: sham (n = 5), ShamT (n = 5), CLP (n = 5) and CLPT (n = 5). The data are presented as the means ± SEM. ∗, p<0.05 vs. sham; #, p<0.05 vs. CLP; and §, p<0.05 vs. ShamT.
The soleus muscle CK activity was higher in the CLP group than in the sham and CLPT groups. However, the plantaris CK activity was higher in the CLP group than in the ShamT and CLPT groups (Figure 4).
The creatine kinase (CK) activity. (A) The soleus and (B) plantaris muscles five days after surgery. The animals were divided into four groups: sham (n = 8), ShamT (n = 8), CLP (n = 6) and CLPT (n = 6). The data are presented as the means ± SEM. ∗, p<0.05 vs. sham; #, p<0.05 vs. CLP; and §, p<0.05 vs. ShamT.
In the soleus and plantaris muscles, TBARS formation was evaluated as an index of lipid peroxidation and carbonyl group levels were evaluated as an index of protein oxidation. As shown in Figures 5-A and 5-C, the TBARS levels in the soleus and plantaris muscles of the rats in the CLP group were significantly increased compared with those of the sham and CLPT groups (p<0.05). Figures 5-B and 5-D demonstrate a greater increase in the carbonyl levels of both muscle types in the CLP group compared with the levels in the sham and CLPT groups (p<0.05).
Oxidative stress parameters. (A, C) Thiobarbituric acid reactive substances (TBARS) and (B, D) carbonyl in the soleus and plantaris muscles at five days after surgery. The animals were divided into four groups: sham (n = 8), ShamT (n = 8), CLP (n = 6) and CLPT (n = 6). The data are presented as the means ± SEM. ∗, p<0.05 vs. sham; #, p<0.05 vs. CLP; and § p<0.05 vs. ShamT.
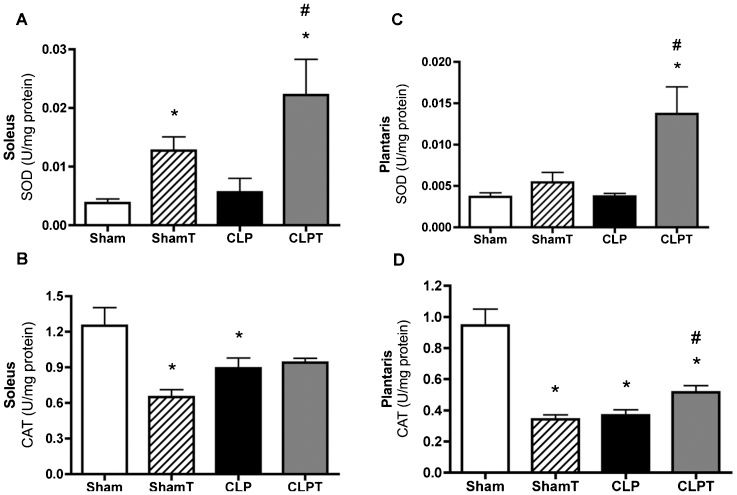
Regulating antioxidant enzymes is important for maintaining the balance between ROS generation and consumption; therefore, the SOD and CAT activity levels were evaluated in the soleus and plantaris muscles. As shown in Figures 6A and 6C the CLP group exhibited no difference in SOD activity compared with the sham group for both muscle types, whereas the CLPT group exhibited a significant increase in SOD activity in both muscle types compared with the sham and CLP groups (p<0.05). However, there was a significant decrease in CAT activity in the CLP group for both muscle types compared with the sham group (p<0.05). In contrast, the CAT activity in the soleus muscles of the CLPT group was similar to that of the sham group, although it differed from that of the CLP group in the plantaris muscle (Figures 6B and 6D).
Antioxidant enzymes. (A, C) Superoxide dismutase (SOD) and (B, D) catalase (CAT) in the soleus and plantaris muscles at five days after surgery. The animals were divided into four groups: sham (n = 8), ShamT (n = 8), CLP (n = 6) and CLPT (n = 6). The data are presented as the means ± SEM. ∗, p<0.05 vs. sham; #, p<0.05 vs. CLP; and § p<0.05 vs. ShamT.
The present study contributes valuable information regarding the effects of aerobic physical preconditioning on different mechanisms that are involved in sepsis-induced skeletal myopathy, including preventing atrophy, lowering lipid peroxidation and protein oxidation, and improving antioxidant defenses.
Several molecular mechanisms of inflammation and cellular damage are involved in the pathogenesis of sepsis, including the excessive generation of ROS (29). These key mediators of cellular injury greatly contribute to the development of sepsis-induced multiple organ dysfunction (29-31). The proinflammatory properties of ROS include endothelial cell damage, chemotactic factor formation, lipid peroxidation and oxidation, neutrophil recruitment, DNA damage, the release of interleukin (IL) and tumor necrosis factor (TNFα), and the formation of peroxynitrite (31). The results of the present study indicated that lipid peroxidation and protein oxidation increased in the oxidative and glycolytic skeletal muscles of rats in the untrained group (CLP) and that tissue damage could be attenuated through exercise training, as observed in the trained group (CLPT) (Figure 5).
In agreement with the results of the present study, some authors have demonstrated that septic patients produce a large amount of ROS without any corresponding antioxidant defenses, which is accompanied by lipid peroxidation (32). Note that one of the mechanisms causing this oxidative stress is the reduction of altered tetravalent oxygen as a consequence of endotoxic, hypoxic, and acidic conditions, which occur in sepsis (14). Thus, in skeletal muscle tissue, acidosis increases the quantity of free iron that is released from myoglobin and hemoglobin (33) thereby activating leukocytes and resulting in superoxide generation (34). Thus, the outcome is a vicious cycle that increases cellular dysfunction, promotes muscle wasting, and is associated with a more serious prognosis (11).
Septic patients frequently develop severe myopathy (2-7) that results in significant weight loss. In the present study, as expected, the CLP group exhibited significant decreases in body weight after 72 hours of sepsis, while the CLPT group exhibited this significant decrease only after 96 hours (Figure 2). Importantly, 120 hours after sepsis induction, the skeletal muscle fiber CSA revealed atrophic conditions only in the soleus and plantaris muscles from the untrained CLP group (Figure 3); in addition, there was significant skeletal muscle injury, as observed by the increase in the TBARS and carbonyl activity (Figure 5). Additionally, there was a significant increase in skeletal muscle anaerobic metabolism in the soleus muscle and a trend toward this increase in the plantaris muscles from rats in the CLP group, as demonstrated by the increase in CK activity (Figure 4). However, in the rats submitted to the aerobic exercise protocol before sepsis induction (CLPT group), this early weight loss was prevented, and skeletal muscle fiber CSA and regular levels of CK, lipid peroxidation and protein oxidation were maintained. These results suggest that the training effects, such as maintaining muscle mass and improving the enzymatic antioxidant system, could be preserved even after 120 hours of sepsis.
It has been shown that exercise training, particularly aerobic exercise, protects skeletal muscles against a variety of stressors, including oxidative stress (15,35). In this context, the elevation of several cytoprotective proteins occurs as a consequence of exercise. For instance, the antioxidant enzymes SOD, CAT and glutathione peroxidase (GPX) in skeletal muscles were shown to be up-regulated (19,36,37) and these enzymes were subsequently able to protect skeletal muscle against ROS-mediated damage (15,35).
The impact of aerobic physical preconditioning on sepsis has not been fully evaluated. Some authors have suggested that 12-wk treadmill training maintains the integrity of the beta-adrenergic receptor adenylate cyclase system, which can be depressed by in vivo endotoxin administration (38). Moreover, Bagby et al. demonstrated that exercise to near exhaustion before intravenous LPS challenge in rats markedly suppressed the systemic TNF response that is normally observed in response to LPS challenge (39). Some studies have shown that appropriate exercise in humans increases anti-inflammatory cytokine IL-6 plasma levels and suppresses the endotoxemia-induced elevation in TNFα (40). For instance, Chen et al. reported that four weeks of exercise training before a sepsis challenge attenuated sepsis-induced systemic hypotension and tachycardia, decreased the number of blood cells, increased the levels of proinflammatory cytokines (TNFα and IL-1β); and, also protected organs from sepsis damage (41). However, there are conflicting studies regarding the effects of transcutaneous electrical muscle stimulation (TEMS) on the quadriceps muscle in septic patients. Gerovasili et al. demonstrated that TEMS can preserve muscle mass; however, Poulsen et al. concluded that the loss of muscle mass was unaffected by TEMS (42,43).
Remarkably, the present study demonstrated that aerobic exercise training was able to prevent atrophy, oxidative stress and muscle damage in skeletal muscle in a rat sepsis model (Figures 3) and 5). The exercise-induced increase in the antioxidant defense system of muscles is most likely the underlying mechanism responsible for protecting muscle cells against oxidative damage. Regarding the removal of ROS from muscle fibers, SOD activity is the first-line of defense against superoxide radicals (15). Subsequently, the present findings show that the ShamT and CLPT groups exhibited significant increases in SOD activity compared with the sham and CLP groups (Figure 6). Other researchers have shown that SOD expression can be induced in skeletal muscle by exercise, which can provide cellular protection against ROS (19,44). For instance, Pinho et al. investigated the effect of 12 weeks of treadmill exercise on the skeletal muscle of rats, and they found that aerobic exercise could increase the SOD/CAT ratio (45).
The results of the present study suggest that exercise increased SOD activity and could be a contributory factor in preventing sepsis-related myocyte injury. CAT, another cellular antioxidant enzyme, is responsible for the catalytic reduction of hydrogen peroxide to water. In the present study, CAT activity levels did not decrease in the CLPT group, whereas a significant decrease in these levels was found in the CLP group compared with the sham group (Figure 6). The trained groups did not exhibit an increase in CAT activity levels, which could be associated with reduced hydrogen peroxide levels due to increased GPX enzyme activity. Moreover, in this study, the CAT activity data from the trained group was in accordance with the results of the study by Powers et al., who showed that there was no change in oxidative and glycolytic muscle CAT activity in response to chronic exercise training (19). Thus, one limitation of the present study is that the exact mechanism that is involved in preventing lipid peroxidation and protein oxidation could not be clarified.
In conclusion, aerobic physical preconditioning prevents atrophy, lipid peroxidation and protein oxidation and improves SOD activity in the skeletal muscle of septic rats. Furthermore, this study suggested that regular exercise may minimize the muscle damage that results from serious bacterial infections.
We thank Hospital Israelita Albert Einstein for covering the publication fees.
No potential conflict of interest was reported.
Coelho CW, Westphal GA, Dal-Pizzol F, Streck EE, and Silva E conceived and designed the study, conducted the experiment, performed the data analysis, reviewed the drafts of the manuscript, and approved the final version of the manuscript. Coelho CW, Jannig PR, Souza AB, and Fronza Jr H conducted the experiment and muscle morphological analysis, reviewed the drafts of the manuscript, and approved the final version of the manuscript. Petronilho F, Constantino L, and Ferreira GK conducted the biochemical analyses, reviewed the drafts of the manuscript, and approved the final version of the manuscript.