Leukocyte-associated immunoglobulin-like receptor-1 is an inhibitory receptor primarily expressed by immune cells. This study was undertaken to define the role of this molecule in osteoclast differentiation and rheumatoid arthritis.
METHODSIn vitro osteoclast assays were performed to characterize the role of Leukocyte-associated immunoglobulin-like receptor-1 in murine and human osteoclastogenesis. Human Leukocyte-associated immunoglobulin-like receptor-1 expression was assessed by immunohistochemistry staining in the synovium of patients with rheumatoid arthritis. The levels of soluble Human Leukocyte-associated immunoglobulin-like receptor-1 were determined by enzyme-linked immunosorbent assay.
RESULTSWe found that multinucleated osteoclast formation from mouse bone marrow cells was inhibited by treatment with a monoclonal antibody against mouse Leukocyte-associated immunoglobulin-like receptor-1 in vitro. By immunohistochemistry, we found that Leukocyte-associated immunoglobulin-like receptor-1 was mainly expressed by macrophages in the inflamed synovial tissue of rheumatoid arthritis patients. In addition, serum and synovial fluid levels of soluble Leukocyte-associated immunoglobulin-like receptor-1 were higher in rheumatoid arthritis patients compared to healthy controls or osteoarthritis patients. Moreover, overexpression of Leukocyte-associated immunoglobulin-like receptor-1 in CD14+ monocytes from healthy volunteers also inhibited human osteoclastogenesis.
CONCLUSIONCollectively, these data demonstrate for the first time that Leukocyte-associated immunoglobulin-like receptor-1 inhibits osteoclastogenesis. Therefore, these results may have therapeutic implications for the treatment of rheumatoid arthritis.
Osteoclasts (OCs), the only cell type capable of resorbing bone, are derived from bone marrow (BM) precursor cells of the monocyte-macrophage lineage (1). The disruption of the dynamic balance of the osteoblasts and OCs leads to pathological bone resorption, such as that observed in rheumatoid arthritis (RA) (2). It is well known that co-stimulatory signals generated by transmembrane immunoreceptors can cooperate to induce OC differentiation. Immunoglobulin (Ig)-like receptors, initially identified and well studied in the immune system, are a novel class of receptors that are critically involved in the regulation of bone homeostasis (3,4). Several studies have specifically suggested critical roles for Ig-like receptors of the leukocyte receptor complex (LRC)-encoded family in the control of osteoclastogenesis, such as the activatory receptors OC-associated receptor (OSCAR) and paired immunoglobulin-like receptor B (PIR-B).
Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is an LRC-encoded transmembrane glycoprotein containing two immunoreceptor tyrosine-based inhibition motifs (ITIMs) in its cytoplasmic tail. LAIR-1 is broadly expressed on cells in the immune system, including natural killer (NK) cells, T cells, B cells, monocytes, and dendritic cells, as well as CD34+ hematopoietic progenitors (5,6). This receptor can inhibit the cytotoxicity of NK cells (activated lymphocyte killer cells) as well as the differentiation of blood precursors into dendritic cells (7–9). Mouse orthologs of human LAIR-1 (hLAIR-1) share ∼40% protein sequence identity and the potent inhibitory capacity of hLAIR-1 (10). Interestingly, collagens are high-affinity ligands for LAIR molecules, and the interaction between LAIR-1 and collagen directly inhibits immune cell activation in vitro and may represent a novel mechanism of peripheral immune regulation mediated by the extracellular matrix (ECM) (11).
The role of the co-stimulatory pathway downstream of immunoreceptor tyrosine-based activating motif (ITAM)-harboring receptors in osteoclastogenesis has been extensively studied. However, the potential implication of ITIM-harboring receptors remains unclear, and the existing literature shows conflicting results. In addition, the involvement of LAIR-1 in OC formation has not yet been studied. In the pathology of RA, chronic inflammation leads to bone destruction (12), and OCs is a key player in this process. For example, in a serum transfer model of inflammatory arthritis, animals that are unable to produce OCs do not show evidence of bone resorption despite the presence of inflammation (13). Therefore, we further investigated the possibility that LAIR-1 may be involved in the pathological process of inflammatory RA by modulating osteoclastogenesis.
MATERIALS AND METHODSEthicsAll procedures were approved by the local ethics committee, and all of the participants provided written informed consent.
Regents and miceAll media components were purchased from GIBCO (Carlsbad, CA, USA). Recombinant cytokines were purchased from R&D Biosystems, Inc. Bovine collagen II and culture-cell BSA and TRAP solutions (No. 387) were purchased from Sigma (St Louis, MO, USA). The functional purified anti-mouse LAIR-1 monoclonal antibodies (mAbs) and isotype control Abs were purchased from eBioscience (San Diego, CA, USA). Human CD14+ monocytes from PBMC were separated using magnetic MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The anti-human antibodies against CD3, CD20, and CD68 were purchased from Maxin Biotechnology (Fuzhou, Fuzhou, China). The anti-mouse LAIR-1 (mLAIR-1) polyclonal antibody, anti-hLAIR-1 antibody (9.1C3), and sandwich ELISA kit for detecting soluble hLAIR-1 were established by our laboratory (14). C57BL/6 mice were purchased from the laboratory animal center at our university. All of the mice were cared for in accordance with the institutional guidelines for animal welfare.
PatientsRA patients were selected at random from the Tangdu Hospital at our university. All of the patients fulfilled the American College of Rheumatology classification criteria for RA and had a disease duration of >1 year. In all the RA patients, disease activity was measured with the Disease Activity Score 28-joint assessment (DAS28) (15). None of the patients were treated with TNF-α blocker therapy. Age- and sex-matched healthy volunteers and osteoarthritis (OA) patients served as controls. A total of 20–30 ml of whole blood was collected by venipuncture for routine laboratory investigations. Sera were isolated from 22 healthy individuals, 18 OA patients, and 17 RA patients. Meanwhile, synovial fluids were treated with hyaluronidase type IV at 20 U/ml (Sigma-Aldrich, St. Louis, MO, USA) for 20 min at 37°C to reduce viscosity. The sera and synovial fluids were stored at -20°C until use. Synovial tissue samples were obtained from RA patients at the time of surgical treatment.
In vitro osteoclastogenesis and mAb stimulationThe induction of murine OCs in vitro was performed as previously described (6). Briefly, total murine BM was flushed from the tibias and femurs of two- to three-week-old mice, and freshly harvested BM cells were cultured at 5×105 cells/ml in minimum essential medium (a-MEM) with 10% FBS containing 10 ng/ml M-CSF. After two days, non-adherent BM cells were discarded, and adherent cells were used as BM monocyte/macrophage lineage cells (BMMs). The BMMs were further cultured for 6 days in the presence of 100 ng/ml recombinant mouse receptor activator of nuclear factor kappa-B ligand (rmRANKL) and 10 ng/ml macrophage colony-stimulating factor (M-CSF) to generate mature OCs. For osteoclastogenesis after mAb stimulation, 96-well flat-bottom plates were coated overnight at 4°C with commercial anti-mLAIR-1 mAbs or control Abs at a concentration of 10 μg/ml in phosphate-buffered saline (PBS). BMMs were plated at 5×105 cells/ml and then cultured as previously described for six days. The medium and factors were replaced after three days. For the human OC cultures, peripheral blood CD14+ monocytes were MACS-sorted from healthy volunteers, according to the guidelines of the ethics committee at our university. TRAP+ multinuclear cells (MNCs, >3 nuclei) were counted.
Flow cytometric (FCM) analysisRabbit polyclonal antibodies against the mLAIR-1 protein were prepared by immunization with the mLAIR-1-Fc fusion protein and were purified from rabbit sera using a Sepharose-4B affinity column (Pharmacia, Peapack, NJ, USA) coupled with mLAIR-1-Fc. To analyze the cell surface expression of mLAIR-1, the cells were incubated with DyLight 649-conjugated anti-mLAIR-1 polyclonal antibody and appropriate isotype controls (Pierce DyLight 649 labeling kit, Thermo scientific, Rockford, IL, USA). The procedures for mouse BM cell, BM-derived macrophage (BM-MΦ), and BM-derived OC (BM-OC) immunofluorescence staining and FCM analysis were conducted as previously described (15). The cells were examined with a flow cytometer (FACS Calibur, BD, San Jose, CA, USA) and analyzed using the WinMDI software ver.2.9 (San Diego, CA, USA).
TRAP stainingOn day six of the BMM culture, the cells were washed with PBS and fixed with 4% paraformaldehyde. The cultures were then stained for TRAP by incubating the cells in a 0.1 M sodium acetate buffer (pH 5.0) containing naphthol AS-BI phosphoric acid sodium salt and fast red ITR salt in the presence of 10 mM sodium tartrate.
Hematoxylin-eosin (HE) and immunohistochemistry (IHC) stainingThe synovial tissue obtained from RA patients was fixed in 10% formalin, embedded in paraffin, and stained with HE. For IHC staining, the Immunocruz staining system (Santa Cruz) was employed according to the manufacturer's instructions. The sections were incubated with anti-hLAIR-1 antibody (9.1C3) or other primary antibodies; negative controls were performed by replacing the primary antibody with normal mouse Ig at the same concentration.
Lentiviral transductionsLenti-X lentiviral expression systems were used to overexpress hLAIR-1 according to the manufacturer's instructions (Clontech, Mountain View, CA, USA). Briefly, the LAIR-1 open reading frame (ORF) was inserted into the Lenti-X plasmid vector and then co-transfected into Lenti-X 293T cells, using a Lenti-X HTX Packaging Mix, to produce recombinant lentivirus for target cell infection, as previously described (16). The resulting lentivirus-containing supernatants were used to infect healthy human CD14+ monocytes for 6–8 hours. The media were then exchanged with fresh α-MEM containing 10% FBS. Infected cells were cultured with 100 ng/ml rhRANKL and 30 ng/ml M-CSF for seven days, and the cells were harvested for assays.
Statistical analysisNormally distributed data were analyzed using the Student's t-test; otherwise, the Wilcoxon signed rank test was used. All data were expressed as the mean ± SEM, and p<0.05 was considered to be statistically significant. All statistical tests were performed with the GraphPad Prism software version 4.0 (GraphPad Software, San Diego, CA, USA).
RESULTSExpression of mLAIR-1 on OCsTo date, the expression of LAIR-1 in the OC system has not been reported. Therefore, we first investigated the expression of LAIR-1 on mature OCs. The rabbit anti-mLAIR-1 polyclonal antibody was generated in our laboratory and used for the FCM analysis. Surface mLAIR-1 expression was predominantly detected on BM-MΦ and was expressed at a lower level by BM-OCs (Figure 1).
Effect of LAIR-1 mAb on OC formationTo investigate the involvement of LAIR-1 in OC formation, we next examined whether treatment with LAIR-1 mAb or collagen in the mono-culture system would affect OC formation. Mouse BMMs were cultured with M-CSF and RANKL for six days in the presence of commercial mLAIR-1 mAb, collagen, or control reagents. Note that we chose bovine collagen II as the ligand for stimulation because the LAIR-1 molecule interacts in a cross-species manner with various collagen molecules and recognizes the conserved Gly-Pro-Hyp repeats present in all collagen trimers. Figure 2A shows representative photographs of the effects of this treatment on TRAP+ multinucleated OC formation. MNC OC formation was significantly decreased in a mono-culture system of BMMs via the cross-linking of mLAIR-1 molecules by anti-mLAIR-1 mAb. These data indicate that LAIR-1 inhibits osteoclastogenesis in vitro (Figure 2B). However, collagen II, a high-affinity ligand for LAIR-1, did not significantly inhibit OC differentiation.
Effect of mLAIR-1 mAb on OC formation induced by soluble RANKL. OC formation was induced by M-CSF (10 ng/ml) and soluble RANKL (100 ng/ml) in the mono-culture system of BMMs. (A) Photomicrographs taken from the cultures stained for TRAP after six days of BM cell culture (magnification, ×100) with the basal medium, normal mouse IgG (mIgG), LAIR-1 mAb, BSA, and collagen. (B) Data are expressed as the number of TRAP+ MNC cells and represent the means ± SEM (n = 3), ∗p<0.01.
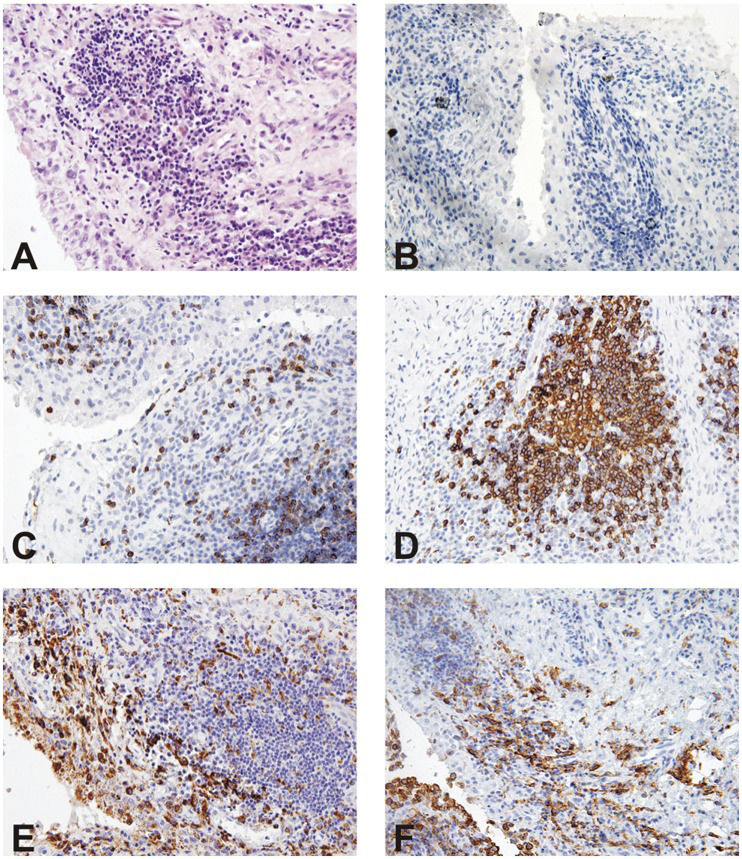
Given the importance of LAIR-1 as a co-stimulatory molecule in murine OC differentiation and function, we investigated the expression pattern of human LAIR-1 in the synovial tissue (Figure 3) of five RA patients by IHC. First, HE staining of the knee joint showed abundant inflammatory cell infiltration at the subsynovial tissues (Figure 3A), whereas positive staining was not observed in sections stained with the isotype control (Figure 3B). Anti-human CD3, CD20, and CD68 antibodies were used to detect the presence of T cells, B cells, and macrophages (Figures 3C-F). At the same time, staining with the anti-hLAIR-1 antibody revealed prominent colocalization with macrophage staining, which indicated (consistent with our previous results) that hLAIR-1 is highly expressed in differentiated mature macrophages.
Human LAIR-1 immunohistochemistry in knee joint sections of RA patients. (C-F) The synovial samples obtained by arthroscopic synovectomy from five RA patients were analyzed by IHC for the expression of CD3, CD20, CD68, and hLAIR-1. (A and B) HE and isotype control staining for IHC are also shown (magnification, ×100).
Because shedding of surface molecules is a commonly observed regulatory phenomenon, we developed a sandwich ELISA to investigate the presence of soluble hLAIR-1 in the serum and synovial fluid. The characteristics of the RA patients are summarized in the supplemental material (Table 1). Of the patients examined, 13 were female and four were male, and the mean patient age was 50.9±17.3 years. The mean age of the healthy volunteers and OA patients was 51.7±13.7 and 58.2±6.4 years, respectively. The mean erythrocyte sedimentation rate (ESR) of the RA patients was 59.3 mm/h, and the mean DAS28 score was 5.5, indicating active disease. Overall, 20 of the 22 healthy volunteers showed detectable levels of serum soluble hLAIR-1 (average 2.72±1.50 ng/ml, range 0.36–6.33 ng/ml). All of the patients with OA or RA were sera-soluble hLAIR-1-positive, with average concentrations of 3.61±1.64 ng/ml (range 0.2–6.33 ng/ml) and 7.15±7.65 ng/ml (range 0.36–30.65 ng/ml), respectively (Figure 4A).
Characteristics of the studied RA patients∗).
| Patient | Age/gender | DAS28 | CRP μg/ml | ESR mm/h | RF U/ml | sCD305 (ng/ml) |
|---|---|---|---|---|---|---|
| 1 | 66/F | 5.2 | 14.3 | 75 | <20.0 | 1.11 |
| 2 | 55/F | 4.0 | 4.9 | 50 | <20.0 | 4.76 |
| 3 | 71/F | 4.1 | 13.8 | 75 | <20.0 | 1.33 |
| 4 | 67/F | 3.8 | 11.7 | 53 | 24.0 | 6.01 |
| 5 | 48/M | 7.0 | 27.6 | 96 | 197.0 | 6.33 |
| 6 | 53/F | 5.6 | 67.5 | 90 | 514.0 | 4.76 |
| 7 | 36/M | 6.9 | <3 | 19 | <20.0 | 2.02 |
| 8 | 72/M | 4.6 | 9.1 | 60 | <20.0 | 4.16 |
| 9 | 43/F | 6.7 | <3 | 73 | 192.0 | 30.65 |
| 10 | 54/F | 7.2 | 5.0 | 106 | 23.3 | 4.76 |
| 11 | 42/F | 4.4 | 4.7 | 26 | <20.0 | 0.36 |
| 12 | 36/F | 7.1 | 66.4 | 52 | 772.0 | 3.87 |
| 13 | 35/F | 3.3 | <3 | 17 | 143.0 | 18.33 |
| 14 | 55/M | 7.3 | 2.3 | 90 | 233.0 | 2.52 |
| 15 | 55/F | 4.9 | 4.1 | 20 | 26.8 | 7.68 |
| 16 | 47/F | 6.6 | 4.7 | 82 | 132.0 | 8.37 |
| 17 | 30/F | 4.7 | <3 | 24 | 76.3 | 14.5 |
Presence of soluble hLAIR-1 in the sera and synovial fluid of patients with RA or OA. Measurements were performed by ELISA, as described in the Materials and Methods. (A) The Wilcoxon signed rank test indicated a statistically significant elevation of soluble hLAIR-1 levels in the sera of RA patients compared to healthy controls, ∗p<0.05. (B) The Wilcoxon signed rank test indicated a statistically significant elevation of soluble hLAIR-1 levels in the synovial fluid of RA patients compared to OA patients, ∗p<0.05.
To explore whether hLAIR-1 is present at sites of inflammation, we measured soluble hLAIR-1 in the synovial fluids of patients with RA (n = 13). Soluble hLAIR-1 levels were compared to those found in the synovial fluids from patients with OA (n = 14) who suffered from joint degeneration with no detectable or mild inflammation, and the soluble hLAIR-1 concentrations in the synovial fluids from RA patients were significantly elevated (average 45.8±24.4 ng/ml, range 13.6–91.2 ng/ml) compared to those in OA patients (average 22.9±13.5 ng/ml, range 9.5–57.3 ng/ml, p<0.05) (Figure 4B). Thus, the increased soluble hLAIR-1 levels in RA synovial fluid seem to reflect the local inflammation in the joints of these patients.
High level of hLAIR-1 expression decreases osteoclastogenesis in healthy humans ex vivoTo further evaluate the significance of these findings in a clinical setting, we also assessed whether the hLAIR-1 molecule could negatively stimulate osteoclastogenesis using monocytes from humans. After culture with 100 ng/ml rhRANKL and 30 ng/ml M-CSF, a decreased number of TRAP+ MNCs were formed when hLAIR-1 lentivirus was added to CD14+ MACS-sorted blood monocytes as compared to the control group, which was infected with a control lentivirus (Figure 5). Furthermore, we failed to detect OC formation when there was no RANKL supplement in the culture medium (vehicle control). Lentivirus infection resulted in an average 89% gene transfer rate, as assessed by PCR (data not shown). These results demonstrated that the over-expression of hLAIR-1 in an in vitro mono-culture system negatively affected osteoclastogenesis. Together, our data reveal that activation of the LAIR-1 pathway inhibits in vitro osteoclastogenesis in both mice and humans.
Human LAIR-1 influences CD14+ monocyte differentiation into mature OCs. Human CD14+ monocytes isolated from healthy volunteers were plated onto a 96-well plate. (A) Preosteoclasts were incubated with M-CSF/RANKL in the absence or presence of hLAIR-1 lentivirus. After cell culturing with RANKL and M-CSF for seven days, TRAP staining was performed. Representative data are shown from three separate experiments, with similar results. (B) TRAP+ MNCs having > three nuclei were counted as OCs, ∗p<0.05.
LAIR-1 is a receptor for ECM collagens, and the interaction between LAIR-1 and collagens is of a high affinity and is dependent on the presence of hydroxyproline in a post-translational modification uniquely present in Gly-Pro-Hyp collagen repeats (20,21). However, when we used collagen II to stimulate mLAIR-1, no significant reduction in the final yield of TRAP+ MNCs was observed. Collagens are the most abundant ECM proteins in most animals, and they can also interact with activatory receptors, such as OSCAR. Therefore, the integral effect of collagens in controlling osteoclastogenesis remains unclear.
It is not known whether the tyrosines in the ITIMs of LAIR-1 become phosphorylated and serve as docking sites for SHP-1 and SHP-2. However, it has been reported that LAIR-1 signaling is not impaired in cells deficient for the SHP-1 and SHP-2 phosphatases; thus, the C-terminal Src Kinase (Csk) may be the key downstream effector of LAIR-1 in cells where phosphatase activity is limited (17). Because Csk has been demonstrated as playing a key role in osteoclastogenesis (although it negatively regulates the kinase activity of c-Src) we hypothesized that LAIR-1 may regulate OC formation through Csk, although this must be further evaluated in subsequent studies (18,19).
Very recently, Meyaard et al. demonstrated that the synovial fluid levels of soluble hLAIR-1 were significantly increased in RA patients and that LAIR-2 (soluble, homologous to hLAIR-1) levels in the urine were significantly correlated with markers of inflammation [23]. Our research group is more interested in the relationship between the level of soluble LAIR-1 and inflammatory arthritis. We found that the sera levels of soluble hLAIR-1 were significantly increased in RA patients, and this result is not fully consistent with Meyaard's work. We think that the reason for this difference may be partially due to the patient samples that we used. All of the patients who participated in this research were never treated with any corticosteroids or biological recombinant medicine, such as adalimumab or etanercept. Moreover, although Meyaard concluded that LAIR-2 was a more efficient antagonist of the LAIR-collagen interaction than soluble LAIR-1, LAIR-1 may also play an important role because the levels of soluble hLAIR-1 are much higher than those of LAIR-2.
It is well known that many trans-membrane receptors can be shed from the cell surface and released into the circulation in soluble form when immune cells are activated. In many cases, the levels of soluble receptors in the circulation can be used as markers of disease severity. Using a sandwich ELISA, we found that the soluble level of hLAIR-1 in sera from healthy individuals was significantly higher than that in RA and OA patients. Furthermore, the soluble hLAIR-1 level in the synovial fluids from RA patients was higher than those in OA patients. These results indicate that increased hLAIR-1 may be released or shed from the cells of RA patients, suggesting that the circulating soluble LAIR-1 may regulate the inhibitory potential of membrane-bound LAIR-1 via competition for ligands. However, the OCs in RA patients may be more prone to induction or activation because of the lower expression of LAIR-1 on the membrane after shedding.
In this article, we demonstrated the negative regulatory role of LAIR-1 in osteoclastogenesis both in humans and in mice. We found that hLAIR-1 is highly expressed by CD68+macrophages from RA patients and that the levels of soluble hLAIR-1 in the sera and synovial fluid from RA patients were significantly greater than those in OA patients, which was also correlated with the levels of rheumatoid factor. Collectively, these results suggest that activated OC differentiation in RA patients may either be caused by the lower expression of hLAIR-1 (shed from the cell membrane) or because of the increased expression of LAIR-1 functional blockers such as soluble hLAIR-1. These findings support evidence for the regulation of OC formation by ITIM-harboring Ig-like receptors, which may have potential clinical significance in RA therapy.
AUTHOR CONTRIBUTIONSZhang Y, Ding Y and Huang Y are the co-first authors. Jin B and Zhuang R are the corresponding authors. All of the authors contributed to the study conception and design, acquisition, analysis and interpretation of the data, manuscript drafting, critical revision for important content, and approval of the final version of the manuscript.
This work was supported by the National Natural Science Foundation of China (No. 30901309 and 30973039).
No potential conflict of interest was reported.