Certain drug classes alleviate the symptoms of Willis-Ekbom's disease, whereas others aggravate them. The pharmacological profiles of these drugs suggest that drugs that alleviate Willis-Ekbom's disease inhibit thyroid hormone activity, whereas drugs that aggravate Willis-Ekbom's disease increase thyroid hormone activity. These different effects may be secondary to the opposing actions that drugs have on the CYP4503A4 enzyme isoform. Drugs that worsen the symptoms of the Willis-Ekbom's disease inhibit the CYP4503A4 isoform, and drugs that ameliorate the symptoms induce CYP4503A4. The aim of this study is to determine whether Saint John's wort, as an inducer of the CYP4503A4 isoform, diminishes the severity of Willis-Ekbom's disease symptoms by increasing the metabolism of thyroid hormone in treated patients.
METHODSIn an open-label pilot trial, we treated 21 Willis-Ekbom's disease patients with a concentrated extract of Saint John's wort at a daily dose of 300 mg over the course of three months.
RESULTSSaint John's wort reduced the severity of Willis-Ekbom's disease symptoms in 17 of the 21 patients.
CONCLUSIONResults of this trial suggest that Saint John's wort may benefit some Willis-Ekbom's disease patients. However, as this trial was not placebo-controlled, the extent to which Saint John's wort is effective as a Willis-Ekbom's disease treatment will depend on future, blinded placebo-controlled studies.
Willis-Ekbom's disease (WED), formerly known as restless legs syndrome, is a common disorder (1–3). In Brazil, the lifetime prevalence of WED is 6.40% (4). Recently (2010), it has been suggested that the WED pathophysiology is caused by an imbalance between the activities of thyroid hormone (TH) and the dopaminergic system (IMBTH/DA (5). This concept is based on the following findings: 1) dopamine (DA) agonists reduce TH levels and alleviate WED symptoms; 2) cytochrome P450 metabolizes TH, and its inhibition (mainly of the CYP3A4 enzyme isoform) aggravates WED symptoms; 3) induction of CYP3A4 alleviates WED symptoms; and 4) DA increases the expression of cytochrome P450, which results in an increase of TH metabolism (6–11). Furthermore, DA also modulates TH by reducing the synthesis of thyroid stimulation hormone (TSH) in the hypothalamus (12).
The major pathway of TH metabolism is through sequential deiodination, but TH is also conjugated with glucuronides and sulfates. Glucuronides are then excreted into bile to act as intermediates in the enterohepatic cycle and fecal excretion of thyroid hormone. Sulfation accelerates the deiodination of different iodothyronines by type I deiodinase and thus initiates the irreversible degradation of the hormone (7). This second pathway is less prevalent and represents approximately 20% of the total TH metabolism (8). TH behaves similarly to a steroid hormone, and most steroid hormones are substrates for the CYP3A4 enzyme isoform. There is strong evidence that when the CYP3A4 isoform is induced, TH glucuronidation and sulfation are also induced (8,9,13,14). Notably, many drugs that relieve or worsen WED symptoms are inducers or inhibitors, respectively, of the CYP3A4 isoform (Table 1).
Suggested mechanisms by which some drugs aggravate WED. The drugs listed in Table 1 (substrates and/or inhibitors of CYP3A4) affect WED by interfering with CYP3A4, thereby decreasing TH metabolism. In addition, some drugs may cause a direct decline in dopaminergic signaling.
| Drug classes | Examples | CYP3A4 substrates | CYP3A4 inhibitors | Effects on DA system |
|---|---|---|---|---|
| Calcium channel blockers | Verapamil | Yes +++ | Yes ++ | No |
| Diltiazem | Yes +++ | Yes ++ | ||
| Selective serotonin reuptake inhibitors | Fluoxetine | Yes + | Yes + | |
| Paroxetine | No | Yes + | No | |
| Sertraline | Yes + | Yes ++ | ||
| Citalopram | Yes +++ | No | ||
| Escitalopram | Yes +++ | No | ||
| Fluvoxamine | No | Yes + | ||
| Neuroleptics | Haloperidol | Yes +++ | Yes ++ | Yes: block DA |
| Chlorpromazine | Yes + | No | D2 receptors | |
| Clozapine | Yes + | Yes + | ||
| Pimozide | Yes +++ | Yes + | ||
| Quetiapine | Yes +++ | No | ||
| Anti-nausea agents | Metoclopramide | No | No | Yes: block DA D2 receptors |
| Lipid lowering agents | Atorvastatin | Yes +++ | Yes + | |
| Lovastatin | Yes +++ | Yes + | No | |
| Simvastatin | Yes +++ | No | ||
| Non-steroidal anti-inflammatory drugs | Diclofenac | Yes + | Yes ++ | No |
| Stimulants | Caffeine | Yes + | Yes ++ | No |
(+ = mild, ++ = moderate, and +++ = strong), References: 9, 13, 14, and 17.
Pramipexole is an effective treatment for WED that is a typical DA agonist with selective affinity for the D2 receptor subfamily (15,16). Pramipexole regulates the expression of many CYP450 enzymes through the tuberoinfundibular and mesolimbic dopaminergic pathways (10,11); furthermore, pramipexole administration decreases the release of TSH (12). These two pharmacological actions of pramipexole may be why it is effective as a WED treatmen. Xenobiotic drugs may also aggravate WED symptoms through decreased dopaminergic signaling (17) (Table 1).
We believe that the non-genomic actions of TH, when unmodulated, underlie WED pathophysiology. TH acts on the mitochondria (18–20), which are responsible for adenosine triphosphate (ATP) production, and it is known that TH increases the number and activity of mitochondria (18). TH has a remarkable range of action in the nervous system, and it is essential for sensory systems (18,21). By increasing the production of ATP, TH increases synaptic transmission by enhancing purinergic neurotransmission (21,22). ATP, which is also a neurotransmitter, is now recognized as having a role in a wide range of nervous system activities, including pain and mechanosensory transduction (22). ATP acts as an excitatory neurotransmitter and is also present in neurons of the dorsal root ganglia (22,23). These neurons have an axon that is connected to peripheral somatosensory receptors (PSS) (23). By enhancing ATP production, TH enhances PSS receptor signaling to the somatosensory cortex; therefore, if modulation does not occur, WED symptoms may ensue. In the so called “forbidden zone for sleep,” the lowest propensity for sleep occurs in the few hours before sleep onset (during the human circadian rhythm) (24,25), concomitantly with a steep increase in the activity (thyrotropin-evening-surge) of the TH axis (26) and an increase in the daily severity of WED symptoms (2,3).
Many medications relieve WED symptoms (17,27), and most of them are able to correct IMBTH/DA directly by inhibiting the TH axis (via CYP450) or indirectly by enhancing dopaminergic signaling (5,17). DA agonists also increase the activity of the diencephalon-spinal DA system, which arises from the dorsal posterior hypothalamic dopaminergic A11 cell group. This system sends projections to the dorsal horn at all spinal levels and to the preganglionic sympathetic neurons (28). There is evidence that this system modulates pain inputs; when dysfunctional, it also may contribute to WED symptoms (28,29).
In somatosensory physiology, PSS receptors in the legs receive information from the environment and convey signals to the somatosensory cortex via the dorsal root ganglia (23), and when this signaling is not appropriately modulated, WED symptoms may ensue. Phantom WED in patients with leg amputation and in WED patients secondary to total knee arthroplasty are examples of the importance of low-threshold sensitivity to stimulation of the peripheral sensory receptors (30–32). On their way to the cortex, the sensory axons synapse in various relay nuclei, in which electrochemical signals are regulated by a number of interspersed neurons (18,23). The primary neural modulation systems are the dopaminergic, GABAergic, glycinergic, and opioidergic systems (18,23). The GABAergic system is the predominant inhibitory system in the CNS (18), and if a drug has the ability to enhance GABA signaling, it may alleviate the WED symptoms. Drugs with this profile include benzodiazepines, benzodiazepine-like drugs, and carbamazepine. Drugs that inhibit the excitability of the sensory neurons that convey messages to the cortex include many anticonvulsant drugs, such as gabapentin, which is an analogue of GABA (27). Clonidine, which is an alpha-2 adrenergic receptor agonist, is a sympatholytic agent that relieves WED symptoms in many patients (27). The beneficial effects of clonidine on RLS symptoms highlight the importance of the connection between the sympathetic system (SS) and the TH axis. The SS has nerve projections to the thyroid gland and induces TH release (33) that, when excessive, may cause IMBTH/DA. Clonidine targets the enhanced functioning of the SS, which makes it a useful drug for alleviating WED symptoms that are occasionally generated in response to an exacerbation of the SS tonus. In iron-deficient patients, WED is more common or symptoms are more severe, and WED patients who receive iron treatment (34,35) often obtain symptom relief. These findings strongly suggest that iron is central to WED pathophysiology. We believe that the role of iron in WED pathophysiology may also be explained by the notion that IMBTH/DA is the main cause of WED.
Iron is a component of many enzymatic systems (18); therefore, it is reasonable to assume that when it is lacking, these enzymes will experience reduced activity levels in accordance with the severity of the iron deficienc. In the chain of reactions that lead to the synthesis of DA, iron is part of the enzyme tyrosine hydroxylase that catalyzes the conversion of tyrosine into L-DOPA. This step in the synthesis of DA is rate-limiting (36); as such, it is possible to infer that iron deficiency may result in decreased production of DA. Additionally, iron is an integral part of all cytochromes (18), including CYP450, which plays a critical role in the degradation of TH. As such, it is reasonable to assume that a lack of iron will upset the balance between TH and DA in two ways: by decreasing DA synthesis and diminishing TH metabolism.
MATERIALS AND METHODSSaint John's wort trialSaint John's wort (SJW) is a standardized extract of hypericum perforatum L that is used to treat mild to moderate depression in adults and children older than six years of age. The usual adult dosage is 300 mg three times daily. SJW is considered to be a fairly safe drug and is devoid of serious side effects (37). SJW is an inducer of the CYP3A4 isoform (13,14) and is also believed to be a mild reuptake inhibitor of the monoamines dopamine, norepinephrine, and serotonin (37). Of the many compounds present in the concentrated extracts of SJW, it is believed that hyperforin is responsible for the induction of the CYP3A4 isoform (37). Compounds present in the hypericum perforatum bush vary according to different circumstances, such as the age of the herb, season of the year, and the different proportions of stalk, leaves, and flowers that are used to prepare the SJW extract (37). SJW acts as a ligand for the nuclear pregnane X receptor to enhance the expression of CYP4503A4 and also stimulates the expression of P-glycoprotein (Pgp) (38), which may result in decreased uptake of many xenobiotics and compounds, including TH. It is known that CYP3A4 and Pgp are coexpressed in many tissues, mainly in the liver and intestinal wall (8).
WED patients (n = 21, 16 female) were told the objective of the trial, and they agreed to volunteer and signed a formal consent for this study, which was approved by the board of ethics on experimentation at our institution, the Faculdade de Medicina de Jundiaí (protocol number 227-2012). All of the studied patients were descendants of Italian and/or Portuguese families. The following inclusion criteria were applied: primary WED sufferers meeting all four criteria for WED according to the IRLSSG (39); experiencing WED symptoms at least three times a week; and having an IRLSSG severity score (39) of at least 15. Additionally, the patients should not have any clinical or therapeutic conditions that may conflict with the experimental drug, such as the use of drugs that are metabolized by CYP4503A4. Exceptions to this latter criterion were three patients who received TH replacement treatment and three patients who were being treated with a low-dosage antidepressant. Thirteen patients were drug-naïve. Eight patients had been taking pramipexole before the trial but had stopped taking the medication for various reasons, although their typical WED paresthesias had been almost entirely alleviated by the use of pramipexole. All of the non-drug-naïve patients stopped taking pramipexole at least three weeks before the SJW trial, and their WED symptoms had returned.
The concentrated extract of SJW used in this trial, which was purchased from Herbarium Laboratórios (Curitiba, Paraná), is marketed in Brazil under the brand name Hipericin (300 mg of SJW dry extract in each capsule). This experiment used only one SJW extract provider; therefore, it was possible to establish that all of the patients would receive equal amounts of the drug. SJW was tested at a daily dose of 300 mg administered two or three hours before bedtime for ten days, during which time the patients received at least three telephone calls to verify treatment compliance. The patients were rated pre- and post-treatment using the IRLSSG severity rating scale (40), which was translated and validated in the Brazilian Portuguese language (41). After ten days of drug administration, the patients were asked about the benefits of the treatment and asked to report, in a face-to-face interview, their subjective impression of the benefit as a percentage of relief in a continuous scale from 0% (no improvement) to 100% (complete improvement). To appraise the study results, SJW was considered effective only if the patient expressed at least 70% relief of their WED symptoms. It was also necessary that the patients subjectively reported an increased quality of sleep during the treatment compared to before the treatment. From the eleventh day onward, patients who were successful in the initial ten-day SJW trial were prescribed the drug at a dosage of 300 mg for three additional months, not on a daily basis but according to their need to achieve relief from their WED symptoms. The patients took the medication for four to five days and then stopped taking it for as long as it would take for the WED symptoms to recur. During the second phase of the trial, the patients received at least one telephone call each week to ascertain their adherence to the treatment. At the completion of the second phase of the trial, the patients were questioned again in a face-to-face or telephone interview regarding their impressions of the ongoing treatment and were also asked if they would be willing to continue with SJW as a treatment for their WED. At the end of the second phase of the trial, all of the patients fulfilled out the IRLSSG rating scale, and the ratings obtained were compared to those taken before the treatment. Statistical analyses were performed using the mean, standard deviation, median, or relative and absolute frequencies. The scores were normally distributed (Kolmogorov-Smirnov test). The paired t-test was used to compare the scores and values before and after the treatment. We assumed p = 0.05 to be significant and used SAS 9.2 software (SAS Institute, Cary, NC, USA) to analyze the results. All of the patients had their TSH, free T4, hemoglobin and ferritin levels determined at the beginning of the trial.
RESULTSThe age of the patients ranged from 12 to 76 years (median 48 years), and their median WED severity score, according to the International Restless Legs Syndrome Study Group (IRLSSG) (39–41), ranged from 17 to 36 (median 24). After the initial ten-day treatment, 17 patients (81%) (14 female) reported experiencing >70% improvement of their WED symptoms and also reported better sleep during the treatment compared to before the treatment (Table 2).
Regarding compliance with the first treatment phase, 11 patients reported that they did not take SJW for one day during the ten day-trial, and four patients did not take SJW on two days of the trial. The age of this successful ten-day treatment group was between 12 and 76 years, with an average of 49.2±17.7 years and a median age of 53 years.
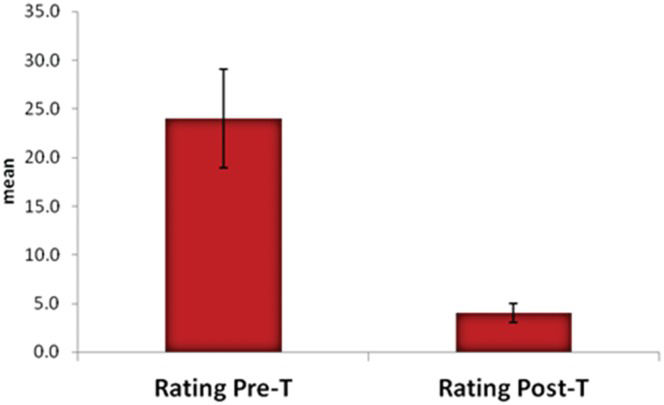
Four patients (two female) reported no improvement during the ten-day trial, all of whom were drug-naïve (ages 21, 42, 45 and 56 years, median WED severity score of 24). Of these four patients, two decided to take pramipexole, after which their WED symptoms were alleviated entirely. Two patients did not return for another interview. All of the patients whose symptoms were alleviated by the ten-day SJW trial agreed to take the drug for three more additional months. All of the patients in this second phase of the trial did not take the drug daily. The patients reported that during the three-month second-phase trial, they took the drug on average for 40 days. They stopped taking the drug for a few days, and then when the WED symptoms returned, they resumed taking the drug for a few days (3-5 days), and so on. Following cessation of the drug, the patients reported that they remained free of WED symptoms for 2-7 days (median 3). After completing the three-month trial, all 17 patients reported a desire to continuously take SJW as a WED treatment. At the beginning of the trial, these 17 patients scored a median of 24 (±5.1) points in the IRLSSG rating severity scale, and after the three-month treatment, the score was 4.1 (±1). When the paired t-test was applied to compare scores and values before and after treatment, p<0.0001 was obtained (Figure 1).
The TSH and free T4 levels measured in all of the patients showed results in the normal reference range for these parameters. None of the patients was anemic, and eight had ferritin levels under 45 μg/L (after the end of the trial they received iron therapy). In this open-label trial, SJW did not cause any side effects in any of the patients.
DISCUSSIONThe results of this pilot trial suggest that SJW may be an effective treatment for some WED patients. However, as this trial was not placebo-controlled, the extent to which SJW is effective as a WED treatment will depend on future, blinded, placebo-controlled studies, which could minimize the placebo effect. We do not advocate that SJW should be considered as a definitive treatment for WED; however, we believe that SJW alleviated WED symptoms of the patients studied because it decreased TH levels by increasing TH degradation through the CYP3A4 enzyme isoform and, perhaps, by increasing Pgp expression. The results of this trial, despite its limitations, seem to add evidence favoring the IMBTH/DA theory as being central to WED pathophysiology. Interfering with the expression of CYP3A4, and perhaps also with Pgp, may decrease the severity of WED symptoms.
Four patients did not experience relief from their WED symptoms, most likely because the extent to which the SJW enhanced their CYP3A4 TH metabolism was not sufficiently high (or the SJW dose used was too small) to decrease TH to lower levels that are compatible with DA counteraction. As explained in the introductory part of this manuscript, there are four pharmacological goals for WED drugs: 1) diminishing the TSH release, 2) enhancing the CYP3A4 expression, 3) modulating the WED sensation inputs on their pathway from the peripheral sensory receptors to the sensory cortex, and 4) diminishing the TH release by the thyroid gland by decreasing the SS activity. SJW most likely relieved the symptoms of the WED patients studied only by enhancing the expression of the CYP3A4 isoform. It is also theoretically possible that SJW alleviated WED symptoms by increasing the activity of the dopaminergic system. However, this specific action of SJW is recognized as mild and therefore most likely did not affect this trial. The IMBTH/DA of the WED patients is most likely subtle. In most WED cases, hyperthyroidism is not more prevalent than it is in normal subjects; therefore, the normal TSH and TH levels observed in all of the patients were not unexpected. During the second phase of the trial, all of the patients were able to become free of WED symptoms for a few days during brief interruptions of the SJW treatment. It is common that the induction or inhibition of CYP450 by xenobiotics lasts for a variable time after exposure to the drug is stopped. Thus, the few days of relief from WED symptoms after the SJW treatment was stopped were not unexpected.
Final considerationsA better understanding of the mechanisms by which drugs ameliorate or worsen WED symptoms may help in the development of new strategies for WED treatment. Furthermore, WED can be considered to be a condition whose severity may be influenced by interactions between xenobiotics and endogenous compounds. For WED patients, the importance of the CYP4503A4 isoform is magnified, and some practical issues may arise. For instance, it may not be advisable for these patients to consume grapefruit juice (popular in the United States) because it is a strong inhibitor of the CYP4503A4 isoform that may thus increase the severity of WED symptoms. Caffeine is also a moderate inhibitor of CYP4503A4; thus, the recommendation of refraining from caffeine after 4 PM might not be sufficient for WED patients because it is possible that the resulting CYP3A4 inhibition will persist well beyond the average half-life of caffeine. As Pgp is able to decrease the TH content of cells, it is reasonable to infer that this protein may also be involved in the fine adjustment of TH physiology. For the treatment of any health problem other than WED, therefore, it would be best for a WED patient to choose a treatment that does not inhibit CYP4503A.
In spite of the inherent limitations of this open-label trial, we hope that its rationale and results may encourage further studies, including larger blind placebo-controlled trials that could demonstrate the extent to which SJW could be used to treat WED.
AUTHOR CONTRIBUTIONSPereira Jr JC conceived and designed the study and was also responsible for writing the manuscript, researching the pertinent literature, and prescribing the drug as well as performing the face-to-face interviews. Pradella-Hallinan M and Alves RC were responsible for revising the manuscript and providing important notes for it.
We are indebted to Prof Karl Ekbom Jr. from the Department of Neurology of Karolinska University, Hospital/Huddinge in Stockholm, Sweden, for his helpful critique of the manuscript and kind assistance. We are also indebted to Prof. Mônica Andersen from the Department of Psicobiologia of the Universidade Federal Paulista in São Paulo, Brazil, for her assistance during the course of the writing of this manuscript.
No potential conflict of interest was reported.