New bone formation is one of the hallmark characteristics of ankylosing spondylitis, which is thereby associated with syndesmophytes. Fetuin-A is a molecule that is abundantly found in calcified tissues and it shows high affinity for calcium phosphate minerals and related compounds. Considering the role of fetuin-A in the regulation of calcified matrix metabolism, we compared the fetuin-A levels in ankylosing spondylitis patients with syndesmophytes with those in patients without syndesmophytes and in healthy controls. We also studied other biomarkers that are thought to be related to syndesmophytes.
METHODS:Ninety-four patients (49 patients without syndesmophytes, 67.3% male, 40.7±8.7 years; 45 patients with syndesmophytes, 71.1% M, 43.9±9.9 years) and 68 healthy controls (44.2±10.6 years and 70.6% male) were included in this study. Syndesmophytes were assessed on the lateral radiographs of the cervical and lumbar spine. The serum levels of fetuin-A, dickkopf-1, sclerostin, IL-6, high-sensitivity C-reactive protein and bone morphogenetic protein-7 were measured with an enzyme-linked immunosorbent assay.
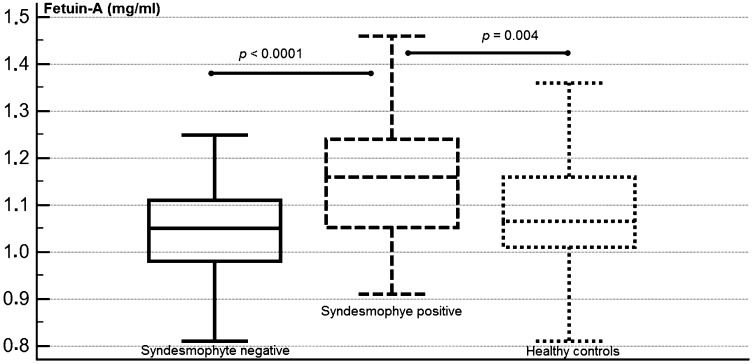
RESULTS:Patients with syndesmophytes had significantly higher levels of fetuin-A compared with patients without syndesmophytes and controls (1.16±0.13, 1.05±0.09 and 1.08±0.13 mg/ml, respectively). However, fetuin-A was not different between the patients without syndesmophytes and controls. Bone morphogenetic protein-7 was significantly lower; dickkopf-1 was significantly higher in patients with ankylosing spondylitis compared with controls. The sclerostin concentrations were not different between the groups. In regression analysis, fetuin-A was an independent, significant predictor of syndesmophytes.
CONCLUSION:Our results suggest that fetuin-A may a role in the pathogenesis of bony proliferation in ankylosing spondylitis.
Ankylosing spondylitis (AS) is a chronic inflammatory disorder that mainly affects the sacroiliac joints and lumbar spine. New bone formation is one of the hallmark characteristics of the disease, which is thereby associated with syndesmophytes and ankylosis 1. Structural damage in the spine is associated with reduced spinal mobility and disability and conventional X-rays of the spine are used for the standard assessment of these changes 2,3. In recent years, there has been considerable interest in the prediction and pathogenesis of syndesmophyte formation. Therefore, several studies have been conducted to identify the factors affecting this process. Today, biomarkers have become a very important field of research in spondyloarthropathy. In this regard, various biomarkers have been used to understand the underlying factors responsible for syndesmophyte formation. However, available data on this subject are still limited and additional information is required to clarify the underlying mechanisms of new bone formation in AS. In a recent study, we reported increased serum fetuin-A levels compared with controls 4. Considering the role of fetuin-A in the regulation of calcified matrix metabolism 5, we designed this study with the primary objective of comparing the fetuin-A levels in AS patients with and without syndesmophytes versus healthy controls. Additionally, we studied other biomarkers that are suggested to be related to the development of syndesmophytes in AS patients.
METHODSStudy population and clinical assessmentThe sample size was calculated with the results of previous studies that investigated the levels of fetuin-A 4, dickkopf-1 (DKK-1) 6 and bone morphogenetic protein-7 (BMP-7) 7 based on α = 0.05 and a power of 80%. At least 39 patients were required per group. We excluded subjects with renal impairment (serum creatinine>1.4 mg/dl) and patients who were treated with glucocorticoids during the previous four weeks. We consecutively enrolled 45 AS patients with syndesmophytes and 49 AS patients without syndesmophytes. All patients met the 1984 modified New York criteria for AS 8. To assess the disease activity, functional ability and spinal mobility, we used the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 9, Functional Index (BASFI) 10 and Metrology Index (BASMI) 11, respectively. There were 68 healthy subjects who served as a control group. The controls were the relatives of the health professionals and blood donors without inflammatory back pain. Data regarding cardiovascular risk factors, such as smoking status (defined as ever/never smokers), hypertension (HT) and diabetes mellitus (DM) were collected. The symptom duration and past or current treatment for AS were also recorded. Local ethical committee approval was obtained and all patients signed informed consent forms.
Radiological evaluationsLateral plain radiographs of the cervical and lumbar spine were used to detect syndesmophytes. The anterior sites of the lower and upper portion of each vertebra were randomly and blindly assessed by two experienced rheumatologists. In discordant cases, radiographs were re-evaluated by both readers together and consensus was reached. The overall kappa value for the inter-examiner agreement for the presence/absence of syndesmophytes was 0.706. The intra-rater agreement for observers 1 and 2 were 0.77 and 0.85, respectively.
Laboratory measurementsFollowing an overnight fast, venous blood samples for laboratory tests were collected between 8:00 and 9:00 AM. Serum samples were preserved at −80°C until assayed. The following enzyme-linked immunosorbent assay (ELISA) kits were applied according to the manufacturers' instructions:
High-sensitivity C-reactive protein (hs-CRP; BioCheck, USA, Cat No: BC-1119): The sensitivity of hs-CRP was 0.1 mg/L. The intra-assay and inter-assay coefficients of variation were 4.44% and 3.28%, respectively.
Interleukin-6 (IL-6; Assay Pro, USA, Cat No: EI1006-1): The minimum detectable dose of IL-6 is ∼0.008 ng/ml. The intra-assay and inter-assay coefficients of variation were 4.9% and 7.5%, respectively.
DKK-1 (Adipo Bioscience, USA, Cat No: SK00312-01): The sensitivity of the assay was 62.5 pg/mL. The intra-assay and inter-assay coefficients of variation were 4-6% and 8-10%, respectively.
BMP-7 (Adipo Bioscience, USA, Cat No: SK00019-01): The sensitivity of the assay was 31.25 pg/mL. The intra-assay and inter-assay coefficients of variation were 4-6% and 8-10%, respectively.
Sclerostin (Biomedica Gruppe, Vienna, Austria, Cat. No.: BI-20492): The sensitivity of the assay was 62.5 pg/mL. The intra-assay and inter-assay coefficients of variation were 4-6% and 8-10%, respectively.
Fetuin-A (Assay Pro, USA, Cat No:EG3501-1): The minimum detectable dose of alpha-2-HS-Glycoprotein is ∼3 ng/ml. The intra-assay and inter-assay coefficients of variation were 5.0% and 7.0%, respectively.
Statistical analysisThe statistical analysis was performed using Statistical Package for the Social Sciences (SPSS), version 16.0 (Chicago, IL, USA). The Kolmogorov–Smirnov normality test was used to determine the distribution pattern of the variables. Most of the parameters, including fetuin-A, DKK-1, sclerostin and hs-CRP, showed a normal distribution and we used parametric tests for the statistical analysis. Data were expressed as the mean ± standard deviation for continuous variables or as percentages of the total for categorical variables. Student's t-test was used for comparisons between two groups of continuous variables. The Pearson χ2 or Fisher's exact test was performed to compare categorical variables. The relationships between different variables were analyzed by the Pearson correlation test. Binary logistical regression analysis was used to identify the factors associated with the presence of syndesmophytes. A one-way ANOVA was used to test for differences among the three group means (patients with and without syndesmophytes and healthy controls). Kappa statistics were used to assess the agreement of the observers. A double-tailed p value of <0.05 was considered statistically significant.
RESULTSThere were 94 patients (49 without syndesmophyte, 67.3% male [M], 40.7±8.7 years; 45 with syndesmophyte, 71.1% M, 43.9±9.9 years) and 68 healthy control subjects (44.2±10.6 years and 70.6% M) in the study group. The age, sex distributions, number of patients with HT, DM and smoking status were similar between the three groups (p>0.05). The demographical and clinical characteristics of the groups are summarized in Table1.
-Demographic and clinical characteristics of the study group.
| AS patients | Controls, n = 68 | p | ||
|---|---|---|---|---|
| Syndesmophyte (+), n = 45 | Syndesmophyte (-), n = 49 | |||
| Age (years) | 43.9±9.9 | 40.7±8.7 | 44.2±10.6 | 0.12 |
| Sex (M/F) | 32/13 | 33/16 | 48/20 | 0.9 |
| Ever smoked, % | 80 | 71.4 | 66.1 | 0.29 |
| Hypertension, % | 6.7 | 2 | 1.5 | 0.26 |
| Diabetes, % | 2.2 | 2 | 0 | 0.48 |
| Disease duration (years) | 15.4±7.5 | 14.7±7.3 | 0.69 | |
| BASFI (0-10) | 3.59±3 | 2.93±2.44 | 0.28 | |
| BASDAI (0-10) | 3.7±2.46 | 4.2±2.6 | 0.41 | |
| BASMI (0-10) | 4.9±1.9 | 3.2±1.5 | <0.0001 | |
| Patients treated with biologics, % | 31.1 | 34.7 | 0.83 | |
| NSAIDs use, % | 75 | 87.8 | 0.18 | |
| Patients treated with SSZ, % | 22.2 | 34.7 | 0.25 |
Continuous data are presented as the mean±standard deviation. BASDAI: Bath ankylosing spondylitis disease activity index, BASFI: Bath ankylosing spondylitis functional index, BASMI: Bath ankylosing spondylitis metrology index, NSAIDs: non-steroid anti-inflammatory drugs and SSZ: sulfasalazine.
Fetuin-A was significantly higher in the AS patients with syndesmophytes compared with patients without syndesmophytes and healthy controls (Figure 1, p<0.05; 1.16±0.13, 1.05±0.09 and 1.08±0.13 mg/ml, respectively). The concentrations of DKK-1 were significantly higher in both the syndesmophyte-positive and -negative patient groups compared with controls (p<0.05; 1911±1344, 1727±1083 and 672±592 pg/ml, respectively). The BMP-7 levels were significantly down-regulated in the patients with and without syndesmophytes compared with healthy controls (p<0.05; 9.4±15.6, 10.7±16.4 and 75.8±110 pg/ml, respectively). Both IL-6 and hs-CRP were significantly higher in the patient group than in controls (p<0.05, Table2). By contrast, the sclerostin levels were similar between the groups (p>0.05). Post hoc comparisons between the groups for each biomarker are summarized in Table2.
-Laboratory characteristics of the study group.
| AS patients | Controls n = 68 | p | p1 | p2 | p3 | ||
|---|---|---|---|---|---|---|---|
| Syndesmophyte (+) n = 45 | Syndesmophyte (-) n = 49 | ||||||
| Fetuin-A (mg/ml) | 1.16±0.13 | 1.05±0.09 | 1.08±0.13 | <0.0001 | <0.0001 | 0.004 | 0.3 |
| DKK-1 (pg/ml) | 1911±1344 | 1727±1083 | 672±592 | <0.0001 | 0.64 | <0.0001 | <0.0001 |
| Sclerostin (pg/ml) | 151±128 | 126±99 | 151±158 | 0.6 | |||
| BMP-7 (pg/ml) | 9.4±15.6 | 10.7±16.4 | 75.8±110 | <0.0001 | 0.99 | <0.0001 | <0.0001 |
| hs-CRP (mg/ml) | 8.9±6.4 | 10.6±7 | 3.3±2.8 | <0.0001 | 0.32 | <0.0001 | <0.0001 |
| IL-6 (pg/ml) | 3.27±0.89 | 3.47±1.26 | 3.04±0.27 | 0.03 | 0.48 | 0.37 | 0.02 |
Continuous data are presented as the mean±standard deviation. DKK-1: dickkopf-1, BMP-7: bone morphogenetic protein-7, hs-CRP: high-sensitivity C-reactive protein.
p represents the significance for the three group comparisons. Post hoc group comparisons were defined as follows: p1: syndesmophyte-positive vs. syndesmophyte-negative patients, p2: syndesmophyte-positive patients vs. healthy controls, and p3: syndesmophyte-negative patients vs. healthy controls.
The disease duration and BASFI and BASDAI values were comparable between the AS patients with and without syndesmophytes (p>0.05); however, the BASMI values were significantly lower in patients with syndesmophytes (p<0.0001, 3.2±1.5 vs. 4.9±1.9). The proportion of patients receiving biological agents, non-steroid anti-inflammatory drugs (NSAIDs) and sulfasalazine (SSZ) were not different between the patients with and without syndesmophytes (p>0.05). The percentages of patients receiving NSAIDs on a continuous or on-demand basis were also similar between the two groups (p>0.05). The fetuin-A levels were significantly higher in patients with syndesmophytes (p = 0.01; 1.16±0.13 vs. 1.05±0.09 mg/ml, respectively) than in those without. The levels of other soluble biomarkers, including DKK-1, sclerostin, BMP-7, IL-6 and hs-CRP, were not different between the two groups (p>0.05).
Correlation analysisCorrelation analysis showed that the presence of syndesmophytes was significantly and positively correlated with the BASMI and fetuin-A levels (p<0.05, r = 0.5 and 0.3, respectively). However, there were no correlations between the presence of syndesmophytes and IL-6, hs-CRP, DKK-1, sclerostin, and BMP-7 levels (p>0.05). Additionally, no correlation was observed for the disease duration, BASDAI, BASFI, anti-tumor necrosis factor alpha (TNF-α) or NSAID therapy, smoking status, presence of HT or DM. Correlations with other variables are given in Table3.
-Correlation coefficients of the clinical and laboratory data.
| Syn | Age | Dd | hs-CRP | IL-6 | Fetuin-A | DKK-1 | Sclerostin | BMP-7 | Smoking | BASFI | BASDAI | BASMI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Syn | 0.3 | 0.5 | |||||||||||
| Age | 0.4 | ||||||||||||
| Dx | 0.4 | 0.3 | |||||||||||
| hs-CRP | 0.6 | 0.3 | -0.2 | 0.3 | 0.4 | ||||||||
| IL-6 | 0.6 | 0.2 | |||||||||||
| Fetuin-A | 0.3 | ||||||||||||
| DKK-1 | 0.3 | 0.2 | |||||||||||
| Sclerostin | 0.2 | ||||||||||||
| BMP-7 | -0.2 | ||||||||||||
| Smoking | |||||||||||||
| BASFI | 0.3 | 0.7 | 0.5 | ||||||||||
| BASDAI | 0.4 | 0.7 | 0.3 | ||||||||||
| BASMI | 0.5 | 0.3 | 0.5 | 0.3 |
The values indicate the correlation coefficients. Note that empty cells indicate that there was no significance between the variables. Syn: syndesmophytes, Dd: disease duration, DKK-1: dickkopf-1, BMP-7: bone morphogenetic protein-7, hs-CRP: high-sensitivity C-reactive protein, BASFI: Bath ankylosing spondylitis functional index, BASDAI: Bath ankylosing spondylitis disease activity index and BASMI: Bath ankylosing spondylitis metrology index.
We performed binary logistic regression analysis to identify the factors that were the independent predictors of syndesmophytes. In the model, we included the age, sex, disease duration, BASDAI, BASFI, BASMI, hs-CRP, IL-6, DKK-1, BMP-7 and fetuin-A. When all predictor variables are considered together, they significantly predict syndesmophytes, X2 = 46.42, df = 12, n = 80 and p<0.001. In the model, increased fetuin-A (odds ratio [OR], and 95% confidence interval [CI] = 422.7, and 4.8-37201, respectively), BASMI (OR, and 95%CI = 3.2, and 1.8-5.9, respectively) and BASDAI (OR, and 95%CI = 0.64, and 0.46-0.89, respectively) significantly and independently predicted syndesmophytes.
DISCUSSIONIn this study, we detected higher fetuin-A levels in AS patients with syndesmophytes compared with patients without syndesmophytes and healthy controls, which was the primary focus of the study. Moreover, fetuin-A was an independent, significant predictor of the presence of syndesmophytes. The results of this study are inconsistent with our previous study, which showed significantly higher levels of fetuin-A in 45 AS patients compared with 29 healthy subjects 4. However, that study did not include a radiographic assessment. In the current study, we excluded subjects with renal failure and patients receiving corticosteroids to avoid the negative effect of these variables on the molecule 12. The age, sex distributions and disease durations as well as the prevalence of hypertension and diabetes mellitus, which may affect the serum fetuin-A levels 13, were similar in the two patient groups. Fetuin-A has also been evaluated in several other inflammatory rheumatic diseases, including rheumatoid arthritis, which revealed decreased levels of this molecule in this group of patients compared with healthy subjects 12. This finding may only be a reflection of the negative acute-phase reactant nature of fetuin-A 14.
New bone formation that develops in both the upper and lower endplates of the vertebra is a hallmark feature of AS. It becomes visible on radiographs as a syndesmophyte, which is the most typical finding of structural damage 1,15. Bony spur formation and ankylosis may limit the range of motion of the spine and eventually compromise function and cause deformity. Therefore, significant research has focused on understanding the cellular and molecular mechanisms of bone formation in AS 3.
Fetuin-A, formerly known as α2-Heremans-Schmid glycoprotein, is a molecule that is mainly synthesized in the liver 12. It is abundantly found in calcified tissues, including bone and ectopic calcified lesions 12,16 and shows high affinity for calcium phosphate minerals and related compounds 17. Animal studies have shown that fetuin-A-deficient mice display calcification of various tissues, suggesting that it may act as an ectopic calcification inhibitor 12,17. However, its abundant presence in bone suggests it may promote bone mineralization. Increased serum fetuin-A levels have been reported in fetal calves compared with adult cows, which may reflect a higher rate of bone mineralization in early fetal life 18,19. Moreover, higher fetuin-A levels were associated with higher bone mineral density among well-functioning community-dwelling elderly women 20. In line with these observations, fetuin-A was demonstrated as both necessary and sufficient for calcification of the type I collagen fibril 21.
DKK-1 is a molecule that has an inhibitory effect on osteoblastic activity by suppressing the WNT signaling pathway. It has been suggested that decreased or dysfunctional DKK-1 is associated with increased osteoblastic activity, inducing or promoting syndesmophytes 22,23. Several reports investigated the DKK-1 levels in AS 24-29. However, these studies are inconsistent in their findings. Some reported increased concentrations of DKK-1 in AS 26,27, whereas others did not find any differences or showed decreased levels of the molecule 28,29. In the current study, we revealed that both the patients with and without syndesmophytes had significantly higher levels of DKK-1 compared with healthy subjects. Sclerostin is a natural inhibitor of the WNT pathway 30. Similar to DKK-1, there are also contradictory results regarding the association of sclerostin and AS 25,29,31,32. In our study, we found that the sclerostin concentrations were not different between the study groups. BMPs are members of the transforming growth factor beta superfamily that play a crucial role in skeletal and joint morphogenesis 33. They are reported to play a role in pathologic new bone formation, especially BMP-7 7. Because of the association of BMPs with new formation, several reports studied different BMPs in AS 7,. In the current study, we showed that the BMP-7 concentrations were significantly lower in the total AS group compared with healthy controls. However, in the subgroup analysis, the BMP-7 levels were not different between the patients with and without syndesmophytes. The main limitations of our study are its cross-sectional design and the lack of information on the HLAB27 status of the patients.
In conclusion, higher fetuin-A levels in AS patients with syndesmophytes than in those without syndesmophytes and in healthy controls suggest a role for this molecule in the pathogenesis of bony proliferation in AS, which needs to be explored in prospective studies.
AUTHORS CONTRIBUTIONTuylu T and Solmaz D helped collecting data from the patients and contributed to the writing. Kozaci D and Gunay N carried out all laboratory analyses in the study. Sari I and Akar S read X-rays and performed the analysis. Sari I was also involved intellectually in the project design and contributed to the discussion. Onen F was involved in the project design and collected the patients' data. Akkoc N helped with the general design of the paper. All authors read and approved the final version of the manuscript.
No potential conflict of interest was reported.