Fetuin-A is a protein secreted from the liver that inhibits arterial calcification deposition and can contribute to insulin resistance. Hyperthyroidism is also associated with insulin resistance. It is not known whether hyperthyroidism has an effect on fetuin-A levels.
METHODSWe measured fetuin-A levels and homeostasis model of assessment-insulin resistance before hyperthyroidism treatment was initiated and after euthyroidism was achieved. A total of 42 patients diagnosed with hyperthyroidism were enrolled in this study. Fetuin-A, insulin, high-sensitivity C-reactive protein, fasting blood glucose, free T3 (fT3), free T4 (fT4), and thyrotropin were measured before and after euthyroidism was established.
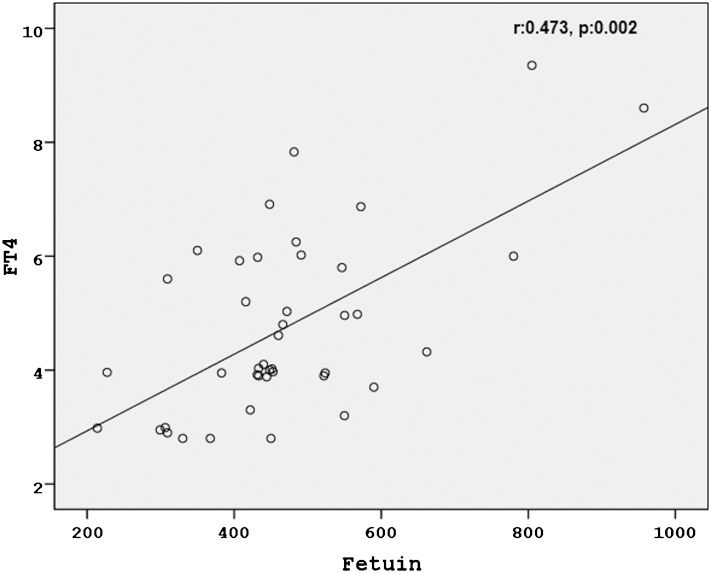
RESULTSBasal fasting blood glucose, high-sensitivity C-reactive protein, insulin, c-peptide, homeostasis model of assessment-insulin resistance, fT3, fT4 and fetuin-A levels were significantly decreased after euthyroidism was achieved (Table 1. Basal fasting blood glucose (r:0.407, p:0.008), high-sensitivity C-reactive protein (r:0.523, p<0.0001), insulin (r:0.479, p:0.001), homeostasis model of assessment-insulin resistance (r:0.541, p<0.0001), fT3 (r:0.492, p:0.001) and fT4 (r:0.473, p:0.002) were positively correlated with basal fetuin-A levels. Basal thyrotropin levels were significantly negatively correlated (r:-0.553, p<0.0001) with basal fetuin-A levels.
CONCLUSIONOur findings suggest that hyperthyroidism influences fetuin-A levels.
Fetuin-A is a protein secreted from the liver that inhibits arterial calcium deposition (1). It interacts with calcium and phosphorus in the serum, increasing their solubility and inhibiting calcium crystal precipitation. Fetuin-A-knockout mice have been shown to develop greater soft tissue calcification compared with wild-type control mice (2-3). Lower fetuin-A levels are associated with cardiovascular disease (CVD) events and all-cause mortality in end-stage renal disease (ESRD) (4).
Fetuin-A inhibits insulin receptor tyrosine kinase activity in the muscle and liver, thereby inhibiting insulin signaling and introducing insulin resistance in vitro (5). In humans, fetuin-A may be an important link between obesity and insulin resistance (6-7). Fetuin-A concentrations are also associated with hepatosteatosis and are elevated in patients with insulin resistance (8). Fetuin-A can also be valuable for the prediction of new-onset type 2 diabetes. In the European Prospective Investigation into Cancer and Nutrition Study, circulating fetuin-A levels predicted the incidence of type 2 diabetes during a 7-yr follow-up (9). In the Health, Aging, and Body Composition study, serum fetuin-A levels were also significantly associated with incident diabetes during a 6-yr follow-up (10).
Hyperthyroidism is also associated with insulin resistance and elevated blood glucose levels (11-12). However, the link between hyperthyroidism and insulin resistance has not been fully elucidated. To our knowledge, fetuin-A levels have not been measured in hyperthyroidism. In this study, we aimed to investigate the level of fetuin-A in hyperthyroidism.
METHODSA total of 42 patients diagnosed with hyperthyroidism were enrolled in this study. Blood samples were collected before hyperthyroidism treatment was initiated and after euthyroidism was achieved. Serum glucose levels were measured with an autoanalyzer with the manufacturer's commercially available kits (Cobas 8000, Roche Diagnostics, Mannheim, Germany). Free T3, free T4, TSH and insulin levels were measured with a chemiluminescence immunometric assay using a UniCell DXI 800 analyzer (Beckman Coulter Inc., Fullerton, CA, USA). Serum hsCRP levels were determined using an ELISA according to the manufacturer's instructions (DRG International, Inc., USA). The intra- and inter-assay coefficients of variation were below 10%. Serum fetuin-A levels were measured with a human fetuin-A ELISA kit (BioVendor Laboratory Medicine, Brno, Czech Republic). The analytical sensitivity of the human fetuin-A ELISA kit was 0.35 ng/mL. The intra- and inter-assay coefficients of variation were below 6.5%. The exclusion criteria were previously known diabetes mellitus, atherosclerotic vascular disease, infections, malignancy, amyloidosis, autoimmune diseases, alcohol consumption or smoking history, congestive heart failure, and liver or renal dysfunction. HOMA-IR was calculated using the following formula:
HOMA-IR = fasting glucose (mmol/L)×fasting insulin (μU/mL)/22.5.
The distribution of continuous variables was determined by the Kolmogorov-Smirnov test. A paired-sample t test was performed to analyze initial and final values with a normal distribution. A Wilcoxon signed rank test was used to analyze data with a skewed distribution. Pearson's and Spearman's analyses were used to identify correlations between study parameters. For all statistics, a two-sided p-value <0.05 was considered statistically significant. All analyses were performed with SPSS 15.0 for Windows.
RESULTSOf the 42 hyperthyroid patients (48.5±15.0 years old), 27 patients were female, and 15 were male. Among the causes of hyperthyroidism, 30 patients had Graves' disease, 11 patients had toxic adenoma, and 1 patient had two toxic nodules. Euthyroidism was achieved in 3.0±0.7 (mean±SD) months. Basal fasting blood glucose, hsCRP, insulin, HOMA-IR, fT3, fT4, calcium and fetuin-A levels were significantly decreased after euthyroidism was achieved (Table 1 (Figure 1. Basal fasting blood glucose (r:0.407, p:0.008), hsCRP (r:0.523, p<0.0001), insulin (r:0.479, p:0.001), HOMA-IR (r:0.541, p<0.0001), fT3 (r:0.492, p:0.001) and fT4 (r:0.473, p:0.002) were positively correlated with basal fetuin-A levels (Figures 2), 3). Basal TSH levels were significantly negatively correlated (r:-0.553, p<0.0001) with basal fetuin-A levels. The basal body mass index (before: 27.1±3.2, after: 28.2±3.7, p<0.0001) increased significantly after euthyroidism was achieved.
Biochemical results before and after euthyroidism was achieved (mean±SD).
| Parameter (reference values) | Hyperthyroid | Euthyroid | p-value |
|---|---|---|---|
| Fetuin-A (ng/mL) | 468.7±143.5 | 234.5±103.8 | <0.0001 |
| FT3 (pg/mL) (2.5-3.9) | 8.6±2.3 | 2.7±0.3 | <0.0001 |
| FT4 (ng/dL) (0.61-1.06) | 4.7±1.6 | 1.2±0.4 | <0.0001 |
| TSH (μIU/mL) (0.41-4.25) | 0.006±0.014 | 0.986±0.467 | <0.0001 |
| Fasting blood glucose (mg/dL) (70-110) | 113.1±15.6 | 94.8±22.2 | <0.0001 |
| Insulin (μU/mL) (1.9-23) | 13.1±2.5 | 8.5±2.1 | <0.0001 |
| HOMA-IR | 3.69±1.07 | 2.00±0.67 | <0.0001 |
| hsCRP (mg/L) (0.068-8.2) | 6.27±2.65 | 1.92±0.79 | <0.0001 |
| Calcium (mg/dL) (8.6-10.2) | 10.1±0.4 | 9.4±0.3 | <0.0001 |
In this study, we found that fetuin-A levels decreased significantly with hyperthyroidism treatment. To our knowledge, this is the first study investigating the effect of hyperthyroidism on fetuin-A levels. In this study, hsCRP levels also decreased significantly with the normalization of thyroid hormones. Furthermore, fetuin-A levels were positively correlated with thyroid hormone, HOMA-IR and hsCRP levels.
Fetuin-A is a liver-derived blood protein that acts as a potent inhibitor of ectopic mineralization. Monomeric fetuin-A protein binds to small clusters of calcium and phosphate. Therefore, fetuin-A is a mineral carrier protein and a systemic inhibitor of pathological mineralization that complements local inhibitors that act in a cell-restricted or tissue-restricted fashion (13). Fetuin-A deficiency is associated with soft tissue calcification in mice and humans. Fetuin-A is a prominent serum glycoprotein as well as a major noncollagenous component of mineralized bone in mammals. In vitro, fetuin-A can inhibit or stimulate osteogenesis, depending on its concentrations (17). Rasul et al. previously found a positive correlation between fetuin-A and bone alkaline phosphatase in type 2 diabetic males. In females, fetuin-A was significantly negatively associated with C-telopeptide levels (18). However, the researchers did not measure thyroid hormones in that study. Thyroid hormones increase bone turnover markers, and hyperthyroidism increases bone turnover. Fetuin-A may be increased in hyperthyroidism through a mechanism related to bone metabolism. Fetuin-A has been studied in several metabolic derangements; however, its role has not been studied in hyperthyroidism. Therefore, we could not compare our results with previous studies.
Sato et al. examined the effects of T3 on the expression of calcification-associated genes and found that a physiological concentration of T3 increased the mRNA level of matrix G1a protein (MGP), which is a potent inhibitor of vascular calcification in vivo (19). They also showed that hyperthyroidism upregulated MGP mRNA 4.5 fold and reduced the calcium content of rat aortic smooth tissue by 11%. Fetuin-A also inhibits tissue calcification in a similar fashion; therefore, we hypothesize that there may be an interaction between fetuin-A synthesis and thyroid hormones because we found a positive correlation between these two parameters. To our knowledge, no experimental study in the literature has investigated the effect of thyroid hormones on the mRNA levels of fetuin-A. Hypercalcemia is a well-known complication of hyperthyroidism, and stimulating fetuin-A synthesis may be an adaptation to prevent ectopic tissue calcification in hyperthyroidism. In a previous study of 32 thyrotoxic teenage girls, 21 (66%) showed some degree of calcification compared with 73 of 600 (12%) in the control group (22). Yamashita et al. previously observed that fibroblast growth factor (FGF)-23 levels (which are also related to soft tissue calcification) decreased significantly with hyperthyroidism treatment (23). However, they did not measure fetuin-A levels, and we therefore cannot compare our results with that study.
Fetuin-A levels have been found to be higher in diabetes and inflammatory diseases, such as ankylosing spondylitis (13). In addition to its functions as an inhibitor of tissue calcification, fetuin-A is an endogenous inhibitor of the insulin receptor tyrosine kinase (14), and fetuin-A-knockout mice exhibit increased insulin sensitivity (15). Recent studies have associated high levels of fetuin-A with an increased risk of type 2 diabetes incidence (16), insulin resistance, hepatosteatosis (8) and metabolic syndrome (7). Li et al. previously showed that fetuin-A plays a protective role in systemic inflammation by activating high-mobility group box 1 (HMGB1) synthesis (20). Hyperthyroidism may also initiate an inflammatory cascade and eventually stimulate fetuin-A synthesis. Insulin resistance in hyperthyroidism may also be related to the increased levels of fetuin-A. Yavuz et al. previously showed that levothyroxine treatment of goiter patients significantly increased hsCRP at 16 weeks (21). In accordance with that study, we found a significant decrease in hsCRP levels with hyperthyroidism treatment. Fetuin-A also has strong anti-inflammatory properties (13). In our study, fetuin-A levels decreased with hsCRP levels, which may also be related to the inflammation-fetuin-A interaction. In our study, insulin levels were significantly positively correlated with fetuin-A levels; furthermore, insulin levels and fetuin-A levels were decreased after euthyroidism was achieved. The relationship between fetuin-A and insulin has been studied in both clinical and experimental studies. Fetuin-A binds to insulin receptors in adipose and muscular tissue and inhibits insulin receptor tyrosine kinase activity as well as insulin receptor autophosphorylation in vivo and in vitro (13). Fetuin-A has also been suggested to potentially cause insulin resistance and metabolic syndrome (13).
In the present study, fasting glucose levels decreased significantly with hyperthyroidism treatment. Fasting glucose levels were significantly positively correlated with fetuin-A levels. It was previously shown that higher fetuin-A concentrations were associated with type 2 diabetes and insulin resistance (24). In another study, fetuin-A levels were significantly correlated with fasting plasma glucose and CRP (25). The present study is in accordance with these findings, as fetuin-A levels decreased significantly as the fasting glucose levels and insulin resistance decreased after hyperthyroidism treatment. Atherosclerosis has been shown to be enhanced in hypothyroidism (26). The anti-atherogenic effects of thyroid hormones may also be related to fetuin-A. Further clinical and experimental studies investigating the effect of hypothyroidism on fetuin-A levels may reveal its influence on atherosclerosis in hypothyroidism.
In conclusion, this study showed that fetuin-A levels declined significantly after hyperthyroidism treatment. Fetuin-A levels were also correlated with thyroid hormones and insulin resistance. Further experimental studies are needed to elucidate the molecular link between the hormones regulating tissue calcification and thyroid hormones.
AUTHOR CONTRIBUTIONSPamuk BO, Ertugrul DT conceived and designed the study, analyzed and interpreted the data, drafted the article, and critically revised the article for important intellectual content. Yılmaz H, Topcuoglu T, Bilgir O, Calan O, Pamuk G organized data in the tables, assisted the patients during the study, helped in the literature revision, drafted the manuscript, and critically revised the manuscript regarding important intellectual content.
No potential conflict of interest was reported.