Forkhead box P3 (FoxP3) expression has been observed in human cancer cells but has not yet been reported in thyroid cells. We investigated the prognostic significance of both FoxP3 expression and intratumoral FoxP3+ lymphocyte infiltration in differentiated thyroid carcinoma cells.
METHODS:We constructed a tissue microarray with 385 thyroid tissues, including 266 malignant tissues (from 253 papillary thyroid carcinomas and 13 follicular carcinomas), 114 benign lesions, and 5 normal thyroid tissues.
RESULTS:We determined the expression of FoxP3 in both tumor cells and tumor-infiltrating lymphocytes using immunohistochemical techniques. Cellular expression of FoxP3 was evident in 71% of benign and 91.9% of malignant tissues. The nuclear and cytoplasmic expression patterns were quantified separately. A multivariate logistic regression analysis indicated that cytoplasmic FoxP3 expression is an independent risk factor for thyroid malignancy. Cytoplasmic FoxP3 staining was inversely correlated with patient age. Nuclear FoxP3 staining was more intense in younger patients and in tumors presenting with metastasis at diagnosis. FoxP3+ lymphocytes were more frequent in tumors smaller than 2 cm, those without extrathyroidal invasion, and in patients with concurrent chronic lymphocytic thyroiditis.
CONCLUSIONS:We demonstrated FoxP3 expression in differentiated thyroid carcinoma cells and found evidence that this expression may exert an important influence on several features of tumor aggressiveness.
Forkhead box P3 (FoxP3) is a member of the forkhead/winged-helix family of transcriptional regulators, which are involved in immune system development and function, notably in the generation of immunosuppressive regulatory T cells (Tregs) (1). The loss of FoxP3 function leads to a reduction in the number of Tregs, resulting in lethal autoaggressive lymphoproliferation, whereas the overexpression of FoxP3 causes severe immunodeficiency (2). Because FoxP3 helps to specify Treg lineages, its expression in primarily lymphoid tissues is expected and has been well documented (3,4). In addition, FoxP3 expression has been observed in human cancer cells, but has not yet been described in thyroid tumors (5).
There is increasing evidence that Tregs also play an important role in cancer immune evasion, which enables tumor cells to elude the host antitumor immune response (6–8). However, the contribution of Tregs to tumor progression and their clinical significance remain poorly understood. Some studies have suggested that the presence of Tregs indicates a worse prognosis (9–11), whereas others have associated their presence with favorable outcomes (12,13). A recent meta-analysis concluded that Treg lymphocytic infiltration was not sufficient to either improve or worsen the prognosis of cancer patients (14).
The existence of an immune response against differentiated thyroid carcinomas has long been recognized (15–17). Previous research has suggested that a local inflammatory response may impede tumor evolution (18) and may indicate a more favorable outcome (15,16). However, a recent study found an association between the presence of Tregs and lymph node metastasis in papillary thyroid carcinomas (19). Unfortunately, the authors did not evaluate the expression of FoxP3 in the analyzed tumor cells.
In this work, we investigated the expression of FoxP3 in malignant and benign thyroid cells. Furthermore, we explored the clinical and pathological roles and the prognostic significance of both FoxP3 expression and intratumoral Treg infiltration in differentiated thyroid carcinomas.
MATERIALS AND METHODSPatientsWe investigated 380 patients, from whom tissue samples were removed and maintained in the tissue bank of the A. C. Camargo Hospital, as summarized in Table 1. Thyroid carcinoma was diagnosed in 266 patients: 253 had papillary thyroid carcinoma (PTC; 153 cases of the classical form, 80 follicular variants and 20 tall cell variants), and 13 had follicular carcinomas (seven minimally invasive and six frankly invasive). In addition, we obtained samples from five normal thyroid tissues and 114 benign thyroid lesions, including 58 nodular goiters and 56 follicular adenomas.
FoxP3 immunostaining and FoxP3+ lymphocytic infiltration in different thyroid tissue subsets.
| Analyzed Groups | Statistical Parameters | Cytoplasmic FoxP3∗) | Nuclear FoxP3∗) | FoxP3+ Lymphocytes |
|---|---|---|---|---|
| Malignant vs. Benign | Accuracy (%) | 61.89 | 50.86 | 38.89 |
| Sensitivity (%) | 62.10 | 50.20 | 11.79 | |
| Specificity (%) | 61.40 | 52.50 | 100.00 | |
| PPV (%) | 79.04 | 72.61 | 100.00 | |
| NPV (%) | 40.86 | 29.59 | 33.45 | |
| p-value | 0.0005 | 0.1514 | 0.0001 | |
| PTC vs. FTC | Accuracy (%) | 59.49 | 53.71 | 69.34 |
| Sensitivity (%) | 72.20 | 59.50 | 0.00 | |
| Specificity (%) | 56.60 | 52.40 | 85.47 | |
| PPV (%) | 27.41 | 21.99 | 0.00 | |
| NPV (%) | 89.97 | 85.16 | 78.61 | |
| p-value | 0.0143 | 0.5937 | 0.0055 | |
| FA vs. FVPTC | Accuracy (%) | 55.69 | 57.41 | 54.72 |
| Sensitivity (%) | 45.70 | 67.30 | 5.88 | |
| Specificity (%) | 64.70 | 48.10 | 100.00 | |
| PPV (%) | 53.85 | 54.98 | 100.00 | |
| NPV (%) | 56.94 | 60.97 | 53.40 | |
| p-value | 0.6885 | 0.1864 | 0.1079 | |
| FA vs. FC | Accuracy (%) | 55.17 | 57.34 | 57.89 |
| Sensitivity (%) | 63.90 | 70.30 | 0.00 | |
| Specificity (%) | 49.00 | 48.10 | 100.00 | |
| PPV (%) | 46.95 | 49.11 | 50.00 | |
| NPV (%) | 65.77 | 69.45 | 57.89 | |
| P-value | 0.6760 | 0.1735 | n.e. |
Abbreviations: FA = follicular adenoma; PTC = papillary thyroid carcinoma; FVPTC = follicular variant of papillary thyroid carcinoma; FC = follicular carcinoma; PPV = positive predictive value; NPV = negative predictive value; n.e. = not evaluated.
Clinical information was obtained from the patients' files. The tumor aggressiveness at diagnosis was ascertained using the Tumor Node Metastasis classification system and the stage classification system for differentiated thyroid carcinomas (20). The patients were followed according to a standard protocol that included periodic total body scans, serum TSH and thyroglobulin (Tg) measurements, X-rays, ultrasonography, computed tomography scans and other procedures as required to detect distant metastases for 12-298 months (mean 43.50±33.29 months; Mo = 21 months). Patients presenting with high non-stimulated serum Tg levels (>2 mg/dl) were subjected to a thorough imaging scan. We used the aforementioned parameters to define tumors as persistent/recurrent and/or presenting with distant metastasis.
Formalin-fixed, paraffin-embedded tissues from all 380 cases were reviewed for diagnostic confirmation and to select the most representative areas for the construction of a tissue microarray (Beecher Instruments®, Silver Springs, MD, USA) for immunohistochemical analysis.
Chronic lymphocytic thyroiditis was investigated in the nonmalignant thyroid parenchyma of the tumor contralateral lobe and was characterized by extensive lymphocytic infiltration with lymphoid follicles, scarring and follicular regenerative activity evidenced by numerous small follicles, which were frequently lined by Hurthle cells (21). The clinical diagnosis of Hashimoto's thyroiditis was confirmed with patient serum thyroperoxidase and thyroglobulin antibody titers.
ImmunohistochemistryThe 5-µm tissue sections intended for microarray construction were placed on electrically charged slides, deparaffinized, and rehydrated with alcohol solutions of decreasing concentration. The endogenous peroxide activity was blocked with H2O2 for 15 min. All the tissue sections were subjected to heat-induced antigen retrieval using a 10% citrate buffer (10 mM, pH 6.0) in a steamer (90°C for 30 min). The tissue sections were then incubated overnight at 6°C with a 1:500 anti-FoxP3 mouse monoclonal antibody (clone 236A/E7; Abcam, Cambridge, UK). The Advance (DAKO, Carpenteria, CA, USA) was used as the reaction detection system. DAB (3.3-diaminobenzidine-tetrahydrochloride; Sigma, St. Louis, MA, USA) was applied as a chromogen for 5 min at room temperature, and the sections were counterstained with hematoxylin. Positive and negative controls were assayed in the same batch of reactions as the patient samples.
Immunohistochemical evaluationThe slides were scored independently by two pathologists (JV and FAS), both of whom were blinded to the tumor features. FoxP3 staining was found in both tumor cells and tumor-infiltrating lymphocytes (Figure 1). To quantify the FoxP3 staining, a tumor cell was considered positive for FoxP3 when an unambiguous brown staining was observed in its cytoplasm or nucleus. Each tissue spot was visually evaluated to estimate the percentage of positive tumor cells and the intensity of the staining. The percentage of positive cells was classified as one of the following: 0 = no positive cells; 1 = up to 10% positive cells; 2 = 10 to 30% positive cells; and 3 = more than 30% positive cells. For statistical purposes, the cases with scores of 0 were defined as negative, and the cases with scores from 1 to 3 were defined as positive.
Different levels of FoxP3 expression in various thyroid tissues and lesions. (A) Normal thyroid tissues showed the lowest FoxP3 staining. (B) Goiter lesions presented with intermediate FoxP3 immunostaining. In contrast to the normal thyroid, the goiter cells exhibited increased cytoplasmic and nuclear staining. (C) Follicular adenoma cells demonstrated pronounced cytoplasmic and nuclear FoxP3 immunostaining. (D) Papillary thyroid carcinomas showed strong FoxP3 expression. In particular, increased cytoplasmic expression is evident. (E) Follicular thyroid carcinoma tissues showed faint immunostaining. (F) A papillary thyroid carcinoma with FoxP3+ tumor cells and FoxP3+ lymphocytes. The white arrows show regulatory T lymphocytes infiltrating the malignant tissue. The black arrows show papillary thyroid carcinoma cells with both cytoplasmic and nuclear FoxP3 staining. Magnification = 400x.
The immunohistochemical expression of FoxP3 was analyzed using the Automated Cellular Imaging System III (ACIS-III) (DAKO, Carpenteria, CA, USA). Cytoplasmic and nuclear staining was assessed separately: tissue spots were digitalized and assigned numerical values proportional to intensity/extension of staining. For the survival analysis, staining ≤ the median was considered negative, and staining > the median was considered positive.
The FoxP3+ lymphocytes were evaluated for each tissue microarray spot individually by estimating the number of positive cells per spot, assuming an approximate area of 0.79 mm2 per cell. To analyze the immunostaining of both the tumor cells and the tumor-infiltrating lymphocytes, aleatory spots were obtained from each tissue, and three spots were assessed to obtain more representative samples from each lesion. For statistical purposes, the cases were grouped into the following categories: 0 (no positive cells), 1+ (up to 10 positive cells per spot) and 2+ (10 or more positive cells per spot).
Statistical analysisThe statistical analyses were performed using the Statistical Analysis System for Windows (SAS Institute Inc., Version 9.1.3, Service Pack 3, 2002-2003, Cary, NC, USA). The disease-free survival was calculated using Kaplan-Meier curves with log-rank comparisons. Nonparametric analyses were performed using the chi-square or Fisher's exact test, as indicated. A multivariate logistic regression model was applied using benignancy/malignancy as the dependent variable and clinical risk factors, including gender and age, as the explicative variables. The Mann-Whitney test was used to compare the continuous or arranged measurements of two groups with variables that did not present a Gaussian distribution, and the Kruskal-Wallis test was used to compare three or more such groups. The immune cell and tumor markers were assessed for their sensitivity, specificity and predictive value in malignancy. Quantitative data were expressed as the mean ± standard deviation. The accuracy of the quantification of the FoxP3 expression in predicting malignancy was evaluated with a receiver operating characteristic (ROC) curve analysis that was based on predicted probabilities from the logistic regression models. All the tests were conducted at a 0.05 significance level.
EthicsThis study was approved by the Research Ethics Committee of the A.C. Camargo Cancer Hospital, São Paulo, Brazil.
RESULTSAs expected, the majority (83.6%) of the patients were females. Individuals with benign (49.2±15.1 years old) and malignant (43.6±15.9 years old) thyroid lesions presented at similar ages at diagnosis. The differentiated thyroid carcinoma patients were classified, according to the pathologic Tumor Node Metastasis staging system (22), as stage I (157 cases), II (28 cases), III (40 cases) or IV (41 cases). Ninety tumors were classified as encapsulated, and 176 were classified as nonencapsulated; 118 were multifocal tumors, and 148 were unifocal tumors. Additionally, 108 patients presented with extrathyroidal invasion, whereas 158 did not. Fifty-five patients (20.6%, including 10 who died from the disease) experienced recurrences, whereas 211 progressed free of disease (79.4%).
Semiquantitative analysis of FoxP3 in tumor cellsFour of the five normal thyroid tissues tested negative for FoxP3 expression, whereas up to 10% of the thyroid cells in the remaining tissue were negative. Eighty-one of the 114 (71.0%) cases of benign lesions, and 244 of the 266 (91.9%) malignant cases were FoxP3 positive (p<0.0001). Twenty-two malignant cases were negative for FoxP3 staining. Fourteen of the 244 FoxP3+ malignant cases were scored as class 1, 11 were scored as class 2 and 219 were scored as class 3. FoxP3 positivity was more frequent among women (95.1%) than men (79.4%, p = 0.0063). There was no association between FoxP3 status and clinical or pathological features of tumor aggressiveness or long-term patient outcome.
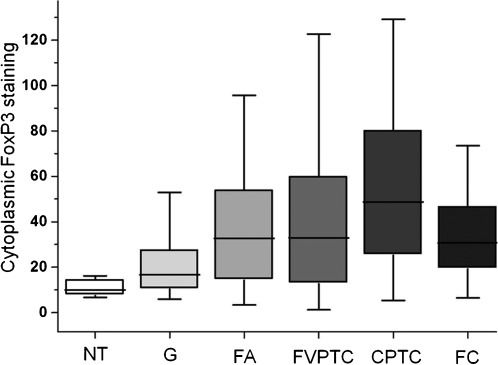
Quantitative analysis of FoxP3 in tumor cellsA close relationship was found between the results of the semiquantitative and quantitative analyses of both the cytoplasmic (p<0.0001) and nuclear FoxP3 staining (p<0.0001); however, the numerical values were not distributed according to a Gaussian curve. We found no significant differences between the cytoplasmic FoxP3 staining levels of the female (52.3±37.4) and male (41.6±40.5, p = 0.0641) differentiated thyroid carcinoma patients. The cytoplasmic FoxP3 levels were inversely correlated with the patient's age at the of the differentiated thyroid carcinoma diagnosis (Spearman r = -0.2913, p = 0.0001). The Kruskal-Wallis test showed distinct patterns of FoxP3 expression among the different types of lesions (p<0.0001), with the malignant lesions staining more intensely (48.1±36.2) than the benign ones (31.9±25.8, p = 0.0005) (Figure 2). The normal thyroid tissues exhibited the lowest cytoplasmic staining (10.9±4.8). The classic papillary thyroid carcinomas received higher staining scores (55.8±38.6) than goiters (22.8±19.6, p<0.0001) and follicular carcinomas (32.5±18.9, p = 0.0143). A multivariate logistic regression model indicated that cytoplasmic FoxP3 expression may be an independent risk factor for malignancy in the diagnosis of thyroid nodules, albeit with a relatively low sensitivity and specificity (Table 1). The differentiated thyroid carcinoma tumors with concurrent chronic lymphocytic thyroiditis exhibited higher cytoplasmic FoxP3 expression (67.6±42.8) when compared with those with no concurrent chronic lymphocytic thyroiditis (46.1 ± 35.6, p = 0.0043).
Cytoplasmic FoxP3 immunostaining in different thyroid tissues. This boxplot of the immunohistochemical quantification data provides evidence of progressive FoxP3 staining in different stages of tumor evolution. Computer-generated numerical values that represent the intensity and extent of the brown (FoxP3) staining are plotted on the y-axis. Abbreviations: NT = normal thyroid; G = goiter; FA = follicular adenoma; FVPTC = follicular variant of papillary thyroid carcinoma; CPTC = classic papillary thyroid carcinoma; FC = follicular carcinoma.
The nuclear FoxP3 staining was also more intense in younger patients (Spearman r = -0.16479, p = 0.01562) but was similar between different types of lesions (p = 0.5622). Aggressive tumors presenting with metastasis at diagnosis had stronger nuclear FoxP3 staining (78.1±24.6) than the less aggressive lesions (63.9±27.6, p = 0.0011). Spearman's log-rank test failed to confirm cytoplasmic (p = 0.72045) or nuclear (p = 0.65969) FoxP3 immunostaining as a prognostic marker.
FoxP3+ lymphocytic infiltrationNo normal thyroid, goiter, follicular adenoma, or follicular carcinoma cases and only 14.5% of the papillary thyroid carcinoma cases presented with FoxP3+ lymphocytes. However, FoxP3 immunostaining again did not prove sufficiently sensitive to be useful as a diagnostic test (Table 1). FoxP3+ lymphocytes were more common in differentiated thyroid carcinomas smaller than 2 cm (p = 0.0078), lacking extrathyroidal invasion (p = 0.0122) and accompanied by chronic lymphocytic thyroiditis (p<0.0001). Tumors with FoxP3+ lymphocytic infiltration also exhibited higher cytoplasmic FoxP3 expression (76.8±43.1) than did those without such infiltration (45.3±33.5, p = 0.0011). However, the nuclear FoxP3 staining was similar among the cases with and without FoxP3+ lymphocytic infiltration (p = 0.95886).
The log-rank test failed to validate FoxP3+ lymphocytic infiltration as a prognostic marker. Likewise, a Cox regression model failed to confirm the reliability of cytoplasmic FoxP3 staining, nuclear FoxP3 staining and FoxP3+ lymphocytic infiltration as independent prognostic markers. Patients with concurrent chronic lymphocytic thyroiditis generated a distinct immune response compared with those without a background of autoimmune disorders. A multivariate analysis that considered chronic lymphocytic thyroiditis and FoxP3+ lymphocytic infiltration as independent variables confirmed that the absence of FoxP3+ lymphocytic infiltration was an independent risk factor for extrathyroidal invasion (p = 0.0048).
DISCUSSIONWe found that FoxP3 is expressed not only in differentiated thyroid carcinoma-infiltrating lymphocytes but also in both the cytoplasm and the nuclei of follicular cells. Our data demonstrated that FoxP3 nuclear expression is related to the aggressiveness of differentiated thyroid carcinomas. In addition, FoxP3+ lymphocytic infiltration was more frequent in tumors smaller than 2 cm, lacking extrathyroidal invasion, and accompanied by chronic lymphocytic thyroiditis.
FoxP3 expression was initially thought to be restricted to hematopoietic cells and tissues; however, recent findings have suggested that other tissues and cell lines are able to express FoxP3 (5),. The roles of Tregs in different tumors remain under dispute. There is evidence that the FOXP3 gene plays a critical role in suppressing pathological transformation in the prostate (26). However, among different prostate samples, we found FoxP3 to be more frequently expressed in tumor cells than in benign nodules, which suggests that the protein may exert different effects in different types of tumors. In fact, FoxP3 expression was shown to progressively decrease as normal cells transform into prostatic intraepithelial neoplasias (PINs) and prostate cancer cells, which implies widespread downregulation that may have occurred at an early stage in prostate cancer development. Conversely, we found the FoxP3 protein to be more highly expressed in carcinomas than in nodular goiters and follicular adenomas.
We found higher cytoplasmic but not nuclear FoxP3 immunostaining in malignant lesions compared with benign nodules, which suggests that the cytoplasmic localization of FoxP3 may be a result of the high mutation rate characteristic of malignant transformation. In fact, the cytoplasmic (rather than nuclear) localization of FoxP3 has been considered a consequence of somatic mutations. The FoxP3 molecule contains a forkhead (FKH)/winged-helix domain that includes a putative nuclear localization signal. Mutations in this domain and other transcriptional or post-transcriptional modifications could generate the cytoplasmic localization of FoxP3 in cancer cells (27–29).
Although FoxP3 expression has also been linked to tumor aggressiveness, the mechanisms of this protein's function and the clinical implications of this association remain unclear. Ban et al. reported a significant association between FOXP3 polymorphisms and susceptibility to autoimmune thyroid disease in Caucasian patients (30). This finding suggests that FoxP3 may be engaged in the regulation of the immune response against thyroid tissue. However, the specific function of FoxP3 in differentiated thyroid carcinomas remains unclear. In addition to provoking a molecular mimicry that enables immune evasion (24), FoxP3 may modulate the patterns of molecular expression in tumor cells, thus favoring an aggressive phenotype (23). However, the relationship between FoxP3 expression and patient prognosis is a matter of debate. In studying HER2-overexpressing breast carcinomas, Ladoire et al. found that FoxP3 expression was an independent prognostic factor for increases in both relapse-free and overall survival (27). On the contrary, Merlo et al. found that the expression of FoxP3 in tumors was inversely associated with patient survival(23). These authors also reported a significant association between FoxP3 expression and lymph node metastasis, suggesting that FoxP3 expression indicated a worse prognosis (23).
Here, we demonstrated higher nuclear, but not cytoplasmic, FoxP3 immunostaining in the more aggressive differentiated thyroid carcinomas presenting with metastasis at diagnosis. The inverse correlations between both cytoplasmic and nuclear FoxP3 levels and age at diagnosis appear to reinforce the finding that FoxP3 expression may be correlated with a worse patient prognosis. However, the log-rank analysis failed to validate FoxP3 as a reliable prognostic marker, supporting the current belief that appropriate management is the most important prognostic factor in differentiated thyroid carcinoma patients. Another noteworthy finding was the correlation between FoxP3 expression and patient age. In fact, although children and adolescents tend to present with higher-stage disease and a greater likelihood of locoregional and distant metastasis, they generally exhibit excellent survival rates (31). In contrast, older patients experience an increased mortality rate in parallel with the occurrence of metastasis (32,33). The intriguing possibility of an association between the inverse correlation between FoxP3 and age revealed in the present work and the clinical behavior of differentiated thyroid carcinoma deserves further study.
We found FoxP3+ lymphocytic infiltration to be associated with the absence of extrathyroidal invasion, a small tumor size and the presence of concurrent chronic lymphocytic thyroiditis. Conversely, studying only 10 papillary thyroid carcinoma cases, French et al. found that regulatory T cell infiltration was closely associated with the presence of lymph node metastasis (19). It is difficult to compare our data on 253 papillary thyroid carcinomas with those obtained by these authors, especially because different methodologies were used. Furthermore, French et al. did not investigate FoxP3 expression in tumor cells.
In conclusion, we demonstrated FoxP3 expression in differentiated thyroid carcinoma cells and found evidence that this expression may exert an important influence on tumor aggressiveness, especially in cases with strong nuclear staining. Larger series of patients are warranted to confirm the clinical utility of FoxP3 staining or Treg infiltration.
AUTHOR CONTRIBUTIONSCunha LL conceived and designed the study, was also responsible for data collection and assembly, data analysis and interpretation, manuscript writing, and final approval of the manuscript. Morari EC contributed to the collection and assembly of data and final approval of the manuscript. Nonogaki S contributed to the data analysis and interpretation, histopathological analysis, and final approval of the manuscript. Soares FA and Vassallo J contributed to the histopathological analysis and final approval of the manuscript. Ward LS contributed to the conception and design of the study, data analysis and interpretation, manuscript writing, and final approval of the manuscript.
We thank Etna Macário and Marcella Lima de Souza for their valuable suggestions and insights.
No potential conflict of interest was reported.