We conducted a study to identify gender differences in factors associated with the first episode of non-adherence in the 12 months following the first antiretroviral prescription.
METHODSA concurrent prospective study of patients initiating antiretroviral therapy in Brazil was conducted from 2001-2002. The self-reported measurement of adherence was defined as an intake of less than 95% of the prescribed number of doses. Only the first occurrence of non-adherence was considered in this analysis. All analyses were stratified by gender. A Cox proportional hazard model was used to estimate the risk of non-adherence, and the time to non-adherence was estimated using the Kaplan-Meier method.
RESULTSThe cumulative incidence of non-adherence was 34.6% (29.7% and 43.9% among men and women, respectively; p = 0.010). Marital status (being married or in stable union; p = 0.022), alcohol use in the month prior to the baseline interview (p = 0.046), and current tobacco use (p = 0.005) increased the risk of non-adherence among female participants only, whereas a self-reported difficulty with the antiretroviral treatment was associated with non-adherence in men only. For both men and women, we found that a longer time between the HIV test and first antiretroviral therapy prescription (p = 0.028) also presented an increased risk of non-adherence.
CONCLUSIONSIn this cohort study, the incidence of non-adherence was 1.5 times greater among women compared to men. Our results reinforce the need to develop interventions that account for gender differences in public referral centers. Additionally, we emphasize that, to achieve and maintain appropriate adherence levels, it is important to understand the barriers to seeking and utilizing health care services.
A broad consensus in the literature supports the necessity of maintaining high rates of adherence to antiretroviral therapy (ART) to achieve viral suppression (1); to prevent the emergence of resistant strains, disease progression and HIV transmission (2–3); and to improve quality of life (4). Despite the necessity of high adherence to ART, findings from a meta-analysis including 84 observational studies suggested that the mean proportion of patients reporting an intake of ≥90% prescribed pills is 62% worldwide (5).
Although it is not completely clear which individual and/or structural factors are associated with greater rates of adherence, evidence from the literature indicates that gender plays an important role in determining differences in HIV therapeutics. For example, women seem to delay initiation of ART more frequently (6), to have higher incidence of treatment interruption (7), to experience more side effects (8), and to have more viral rebound after initial suppression than men (9); furthermore, women appear to metabolize antiretroviral (ARV) drugs differently than men (10).
There is also evidence that women living with HIV face different barriers to adherence than do their male counterparts, including depression, stress, stigmatization, and specific social roles related to gender. Furthermore, researchers suggest that, among females living with HIV, substance abuse and alcohol use are predictive of poor adherence (11–12). However, inconsistencies remain regarding the association between gender and non-adherence. One possible explanation is that this relationship may be confounded by unexamined social or behavioral factors. Furthermore, these associations have not been extensively studied in developing countries (13).
We have previously reported data from a cohort study on adherence to ART (ATAR Project) in which multivariate analyses indicated that unemployment, alcohol use, adverse reactions, number of pills, changes in ART regimen, and a longer duration between the HIV test result and the first prescription were predictors of non-adherence. Gender was associated with non-adherence in the univariate analysis only (14). However, an improved understanding of gender differences in ART adherence may contribute to the development of more effective gender-based interventions that can potentially reduce therapy failure during follow-up. Thus, the aim of this study was to identify gender differences in factors associated with the first episode of non-adherence in the 12 months following the first ARV prescription in AIDS public referral centers in Brazil.
METHODSThis concurrent prospective analysis is part of the ATAR Project, a cohort study conducted during 2001-2002, for which the main objective was to determine the incidence and determinants of non-adherence to ART among people living with HIV (PLHIV). Participants were adult (≥18 years old) patients initiating treatment at two public AIDS referral centers in Belo Horizonte, Brazil, a large urban area with approximately 2.5 million inhabitants (14). Participants were assessed immediately after receiving their first ARV drugs from the pharmacies at each center (baseline interview) and in the first, fourth, and seventh months after initiating therapy (follow-up visits). The maximum follow-up period was 12 months. This analysis aimed at assessing the first episode of non-adherence among patients who returned for at least one follow-up visit during the study period. Ethical approval and consent forms were obtained.
Baseline data included sociodemographic, clinical, and behavioral characteristics, whereas adherence to ART was evaluated at each follow-up visit. Additionally, quality of life and presence of anxiety and depression symptoms were assessed at baseline and at the second follow-up visit.
For the purposes of these analyses, the exposure variables have been grouped as follows: (i) sociodemographics (i.e., age, race, education, marital status, income, job schedule); (ii) behavioral factors (i.e., HIV status disclosure and alcohol, illicit drug, and tobacco use); (iii) health services-related variables (i.e., recruitment site, difficulty in searching for HIV service, use of more than one health service for HIV care, psychological support, and understanding of medical and pharmaceutical counseling related to ARV agents); and (iv) clinical and treatment-related factors (i.e., number of pills per day, self-reported difficulty of ART, clinical classification, TCD4+ lymphocyte count, adverse reactions, time between HIV test result or first medical visit to ARV prescription, self-perceived quality of life, and presence of anxiety and depression symptoms). The clinical and immunological data were extracted from medical charts, which were classified according to the Centers for Disease Control and Prevention definitions (15).
The patients' levels of comprehension of the ARV prescription were assessed using the following six items: drug name, dosage, administration frequency, use precautions or situations demanding special surveillance, side effects, and dietary guidance. Scores indicating the patients' levels of comprehension of their ARV prescriptions were obtained using a latent trait model, estimated with Item Response Theory (IRT) (16), based on the concordance between each patient's answer and the previously published written prescription (17). For this analysis, each score was categorized as sufficient or insufficient, based on the median of participant scores. An understanding of medical or pharmaceutical orientation was self-reported and categorized as follows: sufficient (high or complete), insufficient (median, low or none), or did not receive orientation.
The duration between the initial HIV test and the first ARV prescription and the time between the first medical visit and ARV prescription were categorized using the median points. Age was assessed as a continuous variable. Adverse reactions were defined as any effects or undesirable symptoms reported by the patients over the study period that were attributed to the ARV drug. Symptoms of anxiety and depression were evaluated at baseline using the Hospital Anxiety and Depression Scale (HADS) (18). Quality of life was evaluated using the brief version of the World Health Organization Quality of Life instrument (WHOQOL-bref) (19). We used a global perception of quality of life that classified the participants' quality of life into the following five categories: 'very poor’, 'poor’, 'neither poor nor good’, 'good’, and 'very good’. For the purposes of analysis, the data were dichotomized into two categories, including those who classified their quality of life as 'very poor’, 'poor’, and 'neither poor nor good’, compared to those who reported a 'good’ or 'very good’ quality of life (20).
The self-reported measurement of adherence was defined as the number of prescribed doses of each ARV drug taken during the three days prior to each follow-up visit. Only the first occurrence of non-adherence was assessed, and it was defined as the intake of less than 95% of the prescribed number of doses. All analyses were stratified by gender, and the times to non-adherence were estimated using the Kaplan-Meier method and the Log-rank test.
The strength of association between selected exposure variables and the first episode of non-adherence was estimated by the relative hazard (RH) with 95% confidence interval, obtained from Cox's proportional hazard model for both univariate and multivariate gender-stratified analyses. Variables with p-values equal to or less than 0.20 were included in the final multivariate model with a subsequent sequential deletion. The level of significance required for inclusion in the final model was 0.05 to better ascertain potential confounders. A likelihood ratio test was used to compare all models, and the proportional hazard assumption was assessed by checking the parallelism of the log-log survival curves and the Schoenfeld test.
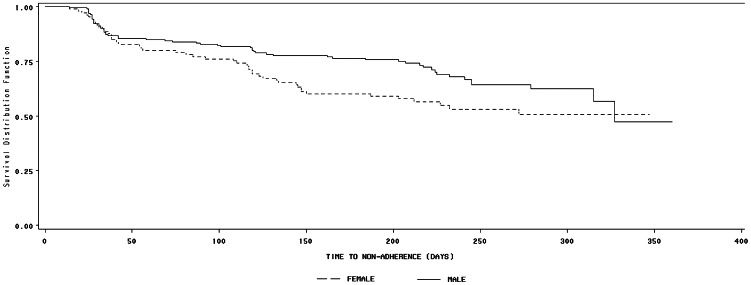
RESULTSAmong 306 participants, 199 (65.0%) were male, and 107 were female. Descriptive characteristics are presented in Table 1. Higher proportions of women reported only basic levels of schooling (41.1%) and unemployment (53.3%) compared to men (19.7% and 26.6%, respectively), whereas men reported a higher proportion of lifetime illicit drug use (38.0%) compared to women (9.0%). There were no marked differences between genders with regard to health service characteristics. However, clinical characteristics indicated that women exhibited a longer duration between the HIV test and the first ART prescription and between the first medical visit and the first ART prescription. Furthermore, women reported less positive self-perceived quality of life (49.5%) than men (62.7%) and higher baseline anxiety (47.0%) and depression symptoms (i.e., moderate-severe) (34.0%) compared to men (29.5% and 15.5%, respectively). The cumulative incidence of non-adherence was 34.6% (95% CI = 29.5%-40.1%), including 29.7% (95% CI = 23.6%-36.3%) among men and 43.9% (95% CI = 34.8%-53.4%) among women (p = 0.01). A significant difference in non-adherence survival Kaplan-Meier curves was identified between men and women (Log-Rank Test: p = 0.038) (Figure 1). The median time to the first episode of non-adherence was a shorter duration for women (272 days) than men (327 days).
Selected descriptive characteristics among 306 participants (199 men and 107 women), Belo Horizonte (MG), 2001-2002.
| Characteristics | Total | Men | Women | |||
|---|---|---|---|---|---|---|
| na | (%) | na | (%) | na | (%) | |
| SOCIODEMOGRAPHICS | ||||||
| Age (years) | ||||||
| >33 | 148 | (48.4) | 99 | (49.8) | 49 | (45.8) |
| ≤33 | 158 | (51.6) | 100 | (50.2) | 58 | (54.2) |
| Race | ||||||
| Non-white | 218 | (74.4) | 137 | (72.5) | 81 | (77.9) |
| White | 75 | (25.6) | 52 | (27.5) | 23 | (22.1) |
| Schooling (years) | ||||||
| ≤4 | 83 | (27.2) | 39 | (19.7) | 44 | (41.1) |
| 4-8 | 85 | (27.9) | 61 | (30.8) | 24 | (22.4) |
| ≥8 | 137 | (44.9) | 98 | (49.5) | 39 | (36.5) |
| Marital status | ||||||
| Single/Divorced/Widowed | 198 | (64.7) | 135 | (67.8) | 63 | (58.9) |
| Married/Stable union | 108 | (35.3) | 64 | (32.2) | 44 | (41.1) |
| Individual income in last month | ||||||
| Yes | 179 | (58.9) | 123 | (62.1) | 56 | (52.8) |
| No | 125 | (41.1) | 75 | (37.9) | 50 | (47.2) |
| Job schedule | ||||||
| Unfixed | 59 | (19.3) | 46 | (23.1) | 13 | (12.1) |
| Fixed | 137 | (44.8) | 100 | (50.3) | 37 | (34.6) |
| Unemployed | 110 | (36.0) | 53 | (26.6) | 57 | (53.3) |
| BEHAVIOR | ||||||
| Disclosure of HIV status | ||||||
| Yes | 255 | (86.4) | 167 | (85.6) | 88 | (88.0) |
| No | 40 | (13.6) | 28 | (14.4) | 12 | (12.0) |
| Alcohol use in month prior to baseline interview | ||||||
| Yes | 111 | (37.6) | 78 | (40.0) | 33 | (33.0) |
| No | 184 | (62.4) | 117 | (60.0) | 67 | (67.0) |
| Current tobacco use | ||||||
| Yes | 101 | (34.2) | 75 | (38.5) | 26 | (26.0) |
| No | 194 | (65.8) | 120 | (61.5) | 74 | (74.0) |
| Lifetime injection drug use | ||||||
| Yes | 17 | (5.8) | 15 | (7.7) | 2 | (2.0) |
| No | 278 | (94.2) | 180 | (92.3) | 98 | (98.0) |
| Lifetime illicit drug use | ||||||
| Yes | 83 | (28.1) | 74 | (38.0) | 9 | (9.0) |
| No | 212 | (71.9) | 121 | (62.0) | 91 | (91.0) |
| HEALTH SERVICES | ||||||
| Recruitment site | ||||||
| CTR/DIP | 248 | (81.1) | 158 | (79.4) | 90 | (84.1) |
| HEM | 58 | (18.9) | 41 | (20.6) | 17 | (15.9) |
| Difficulty in searching for HIV service | ||||||
| Yes | 27 | (9.2) | 20 | (10.3) | 7 | (7.1) |
| No | 266 | (90.8) | 174 | (89.7) | 92 | (92.9) |
| Number of attended HIV health service | ||||||
| Only one | 227 | (77.0) | 151 | (77.4) | 76 | (76.0) |
| More than one | 68 | (23.0) | 44 | (22.6) | 24 | (24.0) |
| Psychological support | ||||||
| Yes | 16 | (5.4) | 10 | (5.1) | 6 | (6.1) |
| No | 278 | (94.6) | 85 | (94.9) | 93 | (93.9) |
| Understanding of medical counseling | ||||||
| Sufficient | 212 | (69.7) | 143 | (72.2) | 69 | (65.1) |
| Insufficient | 76 | (25.0) | 44 | (22.2) | 32 | (30.2) |
| Did not receive | 16 | (5.3) | 11 | (5.6) | 5 | (4.7) |
| Understanding of pharmaceutical counseling | ||||||
| Sufficient | 247 | (80.7) | 160 | (80.4) | 87 | (81.3) |
| Insufficient | 33 | (10.8) | 23 | (11.6) | 10 | (9.4) |
| Did not receive | 26 | (8.5) | 16 | (8.0) | 10 | (9.4) |
| Understanding of ART prescription | ||||||
| Sufficient | 152 | (49.7) | 99 | (49.8) | 53 | (49.5) |
| Insufficient | 154 | (50.3) | 100 | (50.2) | 54 | (50.5) |
| CLINICAL | ||||||
| Number of prescribed pills/day | ||||||
| <7 | 134 | (43.8) | 85 | (42.7) | 49 | (45.8) |
| 7-12 | 122 | (39.9) | 85 | (42.7) | 37 | (34.6) |
| >12 | 50 | (16.3) | 29 | (14.6) | 21 | (19.6) |
| Self-reported difficulty of antiretroviral treatment | ||||||
| Low | 141 | (47.8) | 94 | (48.2) | 47 | (47.0) |
| High | 154 | (52.2) | 101 | (51.8) | 53 | (53.0) |
| Clinical classification | ||||||
| Symptomatic (B-C) | 168 | (56.8) | 110 | (57.3) | 58 | (55.8) |
| Asymptomatic (A) | 128 | (43.2) | 82 | (42.7) | 46 | (44.2) |
| Baseline TCD4+ lymphocyte count | ||||||
| <200/mm3 | 160 | (58.8) | 103 | (59.5) | 57 | (57.6) |
| ≥200/mm3 | 112 | (41.2) | 70 | (40.5) | 42 | (42.4) |
| Number of adverse reactions reported | ||||||
| <3 | 84 | (27.5) | 58 | (29.3) | 26 | (24.3) |
| ≥3 | 221 | (72.5) | 140 | (70.7) | 81 | (75.7) |
| Time between HIV test and first ART prescription | ||||||
| <113 days | 152 | (49.7) | 111 | (55.8) | 41 | (38.3) |
| ≥113 days | 154 | (50.3) | 88 | (44.2) | 66 | (61.7) |
| Time between first medical visit and first ART prescription | ||||||
| <43 days | 144 | (47.1) | 103 | (51.8) | 41 | (38.3) |
| ≥43 days | 162 | (52.9) | 96 | (48.2) | 66 | (61.7) |
| Self-perceived quality of life | ||||||
| Good | 170 | (58.2) | 121 | (62.7) | 49 | (49.5) |
| Poor | 122 | (41.8) | 72 | (37.3) | 50 | (50.5) |
| Baseline anxiety symptoms | ||||||
| None-mild | 189 | (64.5) | 136 | (70.5) | 53 | (53.0) |
| Moderate-severe | 104 | (35.5) | 57 | (29.5) | 47 | (47.0) |
| Baseline depression symptoms | ||||||
| None-mild | 229 | (78.2) | 163 | (84.5) | 66 | (66.0) |
| Moderate-severe | 64 | (21.8) | 30 | (15.5) | 34 | (34.0) |
For men, univariate analyses (Table 2) indicated that non-adherence was associated (p<0.05) with the following factors: alcohol use in the month prior to the baseline interview, lifetime injection drug use, lifetime illicit drug use, self-reported difficulty with ART, baseline TCD4+ lymphocyte count <200/mm3, five or more adverse reactions reported, a longer duration between the HIV test and the first ART prescription, a longer time between the first medical visit and the first ART prescription, poorer self-perceived quality of life, and the presence of moderate/severe symptoms of anxiety or depression. For women, univariate analyses indicated associations (p<0.05) with alcohol use in the month prior to the baseline interview, current tobacco use, and lifetime illicit drug use.
Univariate analysis of the first episode of ART non-adherence among 306 participants (199 men and 107 women), Belo Horizonte (MG), 2001-2002.
| Characteristics | Men | Women | ||||
|---|---|---|---|---|---|---|
| N (%) a | Relative hazard (95% CI) b | p-value | N (%)a | Relative hazard (95% CI) b | p-value | |
| SOCIODEMOGRAPHICS | ||||||
| Age (years) | ||||||
| >33 | 28 (28.3) | 1.00 | 20 (40.8) | 1.00 | ||
| ≤33 | 31 (31.0) | 1.08 (0.65-1.80) | 0.778 | 27 (46.6) | 1.25 (0.70-2.22) | 0.459 |
| Race | ||||||
| White | 14 (26.9) | 1.00 | 8 (34.8) | 1.00 | ||
| Non-white | 43 (31.4) | 1.32 (0.72-2.42) | 0.371 | 37 (45.7) | 1.49 (0.69-3.20) | 0.307 |
| Schooling (years) | ||||||
| ≥8 | 26 (26.5) | 1.00 | 16 (41.0) | 1.00 | ||
| 4-8 | 18 (29.5) | 1.29 (0.71-2.37) | 0.402 | 11(45.8) | 1.28 (0.59-2.76) | 0.531 |
| ≤4 | 15 (38.5) | 1.67 (0.88-3.16) | 0.117 | 20 (45.5) | 1.30 (0.67-2.51) | 0.437 |
| Marital status | ||||||
| Single/Divorced | 42 (31.1) | 1.00 | 23 (36.5) | 1.00 | ||
| Married/Stable union | 17 (26.6) | 0.88 (0.50-1.55) | 0.655 | 24 (54.6) | 1.59 (0.90-2.82) | 0.113 |
| Individual income in last month | ||||||
| Yes | 33 (26.8) | 1.00 | 22 (39.3) | 1.00 | ||
| No | 26 (34.7) | 1.38 (0.83-2.31) | 0.219 | 25 (50.0) | 1.35 (0.76-2.40) | 0.302 |
| Job schedule | ||||||
| Unfixed | 11 (23.9) | 1.00 | 3 (23.1) | 1.00 | ||
| Fixed | 28 (28.0) | 1.23 (0.61-2.48) | 0.555 | 16 (43.2) | 1.58 (0.46-5.43) | 0.468 |
| Unemployed | 20 (37.7) | 1.77 (0.85-3.69) | 0.131 | 28 (49.1) | 2.04 (0.62-6.71) | 0.241 |
| BEHAVIOR | ||||||
| Disclosure of HIV status | ||||||
| Yes | 50 (29.9) | 1.00 | 41 (46.6) | 1.00 | ||
| No | 8 (28.6) | 0.87 (0.41-1.84) | 0.720 | 3 (25.0) | 0.42 (0.13-1.34) | 0.143 |
| Alcohol use in month prior to baseline interview | ||||||
| No | 29 (24.8) | 1.00 | 25 (37.3) | 1.00 | ||
| Yes | 29 (37.2) | 1.73 (1.03-2.89) | 0.038∗ | 19 (57.6) | 1.97 (1.08-3.59) | 0.026∗ |
| Current tobacco use | ||||||
| No | 36 (30.0) | 1.00 | 26 (35.1) | 1.00 | ||
| Yes | 22 (29.3) | 1.12 (0.66-1.91) | 0.672 | 18 (69.2) | 2.53 (1.39-4.63) | 0.003∗ |
| Lifetime injection drug use | ||||||
| No | 50 (27.8) | 1.00 | 42 (42.9) | 1.00 | ||
| Yes | 8 (53.3) | 2.36 (1.12-5.00) | 0.025∗ | 2 (100.0) | 2.73 (0.66-11.3) | 0.166 |
| Lifetime illicit drug use | ||||||
| No | 29 (24.0) | 1.00 | 35 (38.5) | 1.00 | ||
| Yes | 29 (39.2) | 1.91 (1.14-3.21) | 0.014∗ | 9 (100.0) | 3.33 (1.58-6.98) | 0.002∗ |
| HEALTH SERVICES | ||||||
| Recruitment site | ||||||
| CTR/DIP | 52 (32.9) | 1.00 | 39 (43.3) | 1.00 | ||
| HEM | 7 (17.1) | 0.55 (0.25-1.21) | 0.135 | 8 (47.1) | 1.31 (0.61-2.81) | 0.488 |
| Difficulty in searching for HIV service | ||||||
| No | 48 (27.6) | 1.00 | 43 (46.7) | 1.00 | ||
| Yes | 10(50.0) | 1.90 (0.96-3.77) | 0.066 | 1 (14.3) | 0.23 (0.03-1.63) | 0.140 |
| Number of attended HIV health service | ||||||
| Only one | 47 (31.1) | 1.00 | 36 (47.4) | 1.00 | ||
| More than one | 11 (25.0) | 0.74 (0.39-1.43) | 0.376 | 8 (33.3) | 0.57 (0.27-1.24) | 0.156 |
| Psychological support | ||||||
| Yes | 2 (20.0) | 1.00 | 1 (16.7) | 1.00 | ||
| No | 56 (30.3) | 1.63 (0.40-6.71) | 0.497 | 43 (46.2) | 3.12 (0.43-22.7) | 0.261 |
| Understanding of medical counseling | ||||||
| Sufficient | 42 (29.4) | 1.00 | 27 (39.1) | 1.00 | ||
| Insufficient | 15 (34.1) | 1.03 (0.57-1.86) | 0.920 | 16 (50.0) | 1.36 (0.73-2.52) | 0.335 |
| Did not receive | 2 (18.2) | 0.62 (0.15-2.56) | 0.507 | 4 (80.0) | 2.33 (0.81-6.68) | 0.116 |
| Understanding of pharmaceutical counseling | ||||||
| Sufficient | 48 (30.0) | 1.00 | 39 (44.8) | 1.00 | ||
| Insufficient | 6 (26.1) | 0.70 (0.30-1.64) | 0.416 | 5 (50.0) | 1.02 (0.40-2.59) | 0.970 |
| Did not receive | 5 (31.3) | 1.04 (0.41-2.62) | 0.932 | 3 (30.0) | 0.63 (0.19-2.03) | 0.435 |
| Understanding of ART prescription | ||||||
| Sufficient | 30 (30.3) | 1.00 | 20 (37.7) | 1.00 | ||
| Insufficient | 29 (29.0) | 0.98 (0.58-1.63) | 0.923 | 27 (50.0) | 1.47 (0.82-2.62) | 0.194 |
| CLINICAL | ||||||
| Number of prescribed pills/day | ||||||
| <7 | 20 (23.5) | 1.00 | 18 (36.7) | 1.00 | ||
| 7-12 | 28 (32.9) | 1.35 (0.76-2.41) | 0.308 | 17 (46.0) | 1.15 (0.59-2.24) | 0.674 |
| >12 | 11 (37.9) | 1.41 (0.67-2.94) | 0.363 | 12 (57.1) | 1.61 (0.77-3.34) | 0.204 |
| Self-reported difficulty of antiretroviral treatment | ||||||
| Low | 15 (16.0) | 1.00 | 19 (40.4) | 1.00 | ||
| High | 44 (43.6) | 2.89 (1.61-5.20) | <0.001∗ | 26 (49.1) | 1.26 (0.70-2.27) | 0.447 |
| Clinical classification | ||||||
| Symptomatic (B-C) | 30 (27.3) | 1.00 | 21 (36.1) | 1.00 | ||
| Asymptomatic (A) | 29 (35.4) | 1.35 (0.81-2.26) | 0.247 | 23 (50.0) | 1.50 (0.83-2.72) | 0.176 |
| Baseline TCD4+ lymphocyte count | ||||||
| < 200/mm3 | 19 (18.5) | 1.00 | 24 (42.1) | 1.00 | ||
| ≥ 200/m m3 | 29 (41.4) | 2.56 (1.44-4.58) | 0.002∗ | 19 (45.2) | 1.11 (0.61-2.03) | 0.741 |
| Number of adverse reactions reported | ||||||
| <5 | 20 (18.4) | 1.00 | 20 (40.0) | 1.00 | ||
| ≥5 | 38 (42.7) | 2.30 (1.33-3.95) | 0.003∗ | 27 (47.4) | 1.20 (0.67-2.14) | 0.543 |
| Time between HIV test and first ART prescription | ||||||
| <113 days | 23 (20.7) | 1.00 | 13 (31.7) | 1.00 | ||
| ≥113 days | 36 (40.9) | 2.55 (1.49-4.37) | 0.001∗ | 34 (51.5) | 1.81 (0.96-3.44) | 0.068 |
| Time between first medical visit and first ART prescription | ||||||
| < 43 days | 23 (22.3) | 1.00 | 15 (36.6) | 1.00 | ||
| ≥ 43 days | 36 (37.5) | 1.90 (1.13-3.21) | 0.016∗ | 32 (48.5) | 1.41 (0.76-2.60) | 0.275 |
| Self-perceived quality of life | ||||||
| Good | 29 (24.0) | 1.00 | 23 (46.9) | 1.00 | ||
| Bad | 29 (40.3) | 1.87 (1.12-3.14) | 0.017∗ | 20 (40.0) | 0.81 (0.44-1.48) | 0.491 |
| Baseline anxiety symptoms | ||||||
| None-mild | 33 (24.3) | 1.00 | 21 (39.6) | 1.00 | ||
| Moderate-severe | 25 (43.9) | 1.93 (1.15-3.25) | 0.013∗ | 23 (48.9) | 1.27 (0.70-2.30) | 0.425 |
| Baseline depression symptoms | ||||||
| None-mild | 42 (25.8) | 1.00 | 27 (40.9) | 1.00 | ||
| Moderate-severe | 16 (53.3) | 1.95 (1.10-3.47) | 0.023∗ | 17 (50.0) | 1.36 (0.74-2.50) | 0.322 |
The final multivariate model is shown in Table 3. The results indicated that self-reported difficulty of ART (p = 0.002), five or more adverse reactions reported (p = 0.007), and a longer duration between the HIV test and the first ART prescription (p = 0.002) were independently associated with increased risk of non-adherence among male participants. In contrast, being married or in a stable union (p = 0.022), alcohol use in the month prior to the baseline interview (p = 0.046), current tobacco use (p = 0.005), and a longer duration between the HIV test and the first ART prescription (p = 0.028) were associated with increased risk of non-adherence among female participants.
Relative hazard with 95% confidence interval obtained from multivariate analysis of the first episode of ART non-adherence among 306 participants (199 men and 107 women), Belo Horizonte (MG), 2001-2002.
| Men | Women | |||
|---|---|---|---|---|
| Characteristics | Relative hazard (95% CI) | p-value | Relative hazard (95% CI) | p-value |
| Marital status | ||||
| Single/Divorced | - | 1.00 | ||
| Married/Stable union | - | - | 2.01 (1.10-3.64) | 0.022 |
| Alcohol use in month prior to baseline interview | ||||
| No | - | 1.00 | ||
| Yes | - | - | 1.86 (1.01-3.43) | 0.046 |
| Current tobacco use | ||||
| No | - | 1.00 | ||
| Yes | - | - | 2.40 (1.30-4.41) | 0.005 |
| Self-reported difficulty of antiretroviral treatment | ||||
| Low | 1.00 | - | ||
| High | 2.53 (1.40-4.56) | 0.002 | - | - |
| Number of adverse reactions reported | ||||
| <5 | 1.00 | - | ||
| ≥5 | 2.11 (1.22-3.63) | 0.007 | - | - |
| Time between HIV test and first ART prescription | ||||
| <113 days | 1.00 | 1.00 | ||
| ≥113 days | 2.38 (1.37-4.12) | 0.002 | 2.22 (1.09-4.51) | 0.028 |
In this analysis, the high incidence of non-adherence was 1.5 times greater among women compared to men. This finding is consistent with studies reporting poorer adherence among women that have significant differences identified between genders (2),. However, other studies have failed to find a significant association between gender and ARV adherence (11–12), and yet another study reported higher adherence rates among women (13). These discordant results may be due to methodological distinctions, sample differences, or sociocultural divergence.
A literature review conducted on the adherence to ART in developed countries and spanning from 2000-2011 was conducted to determine whether the prevalence of non-adherence varied by gender (24). The authors found that, when using higher cutoff points (100% and 95%), women exhibited a lower mean proportional adherence than men across all measurements. In contrast, lower cutoff points (90% and 80%) indicated that women reported a higher mean proportional adherence across the majority of measurements. Although the authors concluded that these findings suggested that female gender status often predicts lower adherence, they emphasized that differences in methodology may account for these contradictory results.
Distinct gender differences were observed in our results. The only variable represented in the multivariate model for both men and women was the association of non-adherence with a longer duration between the HIV test result and the first ARV prescription. This delay in treatment initiation is concerning and can be the result of either the patient's difficulty in accepting the diagnosis and beginning treatment or a reduced perception of the necessity of treatment due to a lack of symptoms. The follow-up protocol for individuals living with HIV requires that they return to the health service for medical consultations every six months on average. Moreover, HIV referral services tend to be overloaded with patients, and priority is usually given to those with symptoms or more severe clinical conditions (25).
However, people living with HIV who are asymptomatic tend to have less awareness of the disease and may not perceive the seriousness of the AIDS threat (26). This perception potentially results in poor and delayed searches for care, despite the established capabilities and significant investments made by the Brazilian Health System to help diagnose, treat, and care for people living with HIV and AIDS. We emphasize the need to implement counseling and create alternative facilities for ambulatory care and treatment (27).
Among men, self-reported difficulty with ART was a predictor of non-adherence. To acquire a behavior, such as adherence to ARV therapy, an individual needs to believe that benefits of the behavior compensate for the potential difficulties (28).
Consistent with the literature, our results indicated that clinical factors such as adverse reactions have been consistently associated with non-adherence (5). Adverse reactions are common with ARV therapy, especially among patients initiating treatment (3,10–12,22),.
Among female participants, we determined that marital status, including being married or in a stable union, was associated with poor adherence. Potential explanations for this finding include differences in health service attendance given the responsibility to manage one's home and children (30). Health professionals from HIV referral services must understand and involve the family and nurture social relationships, with the aim of achieving and maintaining high levels of adherence to ART for this particular population (31). In addition to biological differences, women with HIV often balance multiple roles, have limited access to healthcare, report less household income, and express other concerns that are not typically shared by HIV-positive men (32).
Finally, our results emphasized that the behavioral characteristics that can be modified after the initiation of ART may be critical for the early adoption of adherent behavior. The analysis indicated that, among women, alcohol use was significantly associated with non-adherence. These findings are consistent with the literature (11–12), thereby suggesting that alcohol use may disproportionately impact ARV adherence in women. Furthermore, alcohol use is highly prevalent among individuals with HIV/AIDS (21,31). A systematic review of the literature indicated that, among individuals with HIV/AIDS, alcohol use disorders are related to decreased ART adherence and to poorer HIV treatment outcomes (31). Other studies support the correlation with alcohol dependence in women, which is thought to partially reflect the relative behavior frequency in relation to male participants (11–12). A separate study conducted between 1999 and 2004 using two cohorts of women also indicated that alcohol use (including binging and heavy and low consumption) reduced adherence (33).
Our findings also indicated that tobacco use among women was associated with non-adherence. This result is corroborated by the literature, which has highlighted that increased tobacco consumption soon after initiation of ART was related with subsequent non-adherence (34). Similarly, nicotine dependence appears to be related to non-adherence in the context of other substance use behavior, and depression may, in part, mediate the relationship between tobacco use and ART non-adherence (34–35). Thus, in addition to other health implications due to tobacco use, particularly in women, more interventional studies are needed to focus on smoking and non-adherence.
In conclusion, this gender analysis indicated that social, behavioral, clinical, and health service characteristics associated with non-adherence at the beginning of ART are potentially different among women and men. Whereas among men, these factors directly related to the treatment per se, i.e., adverse reactions and self-reported difficulty, the non-adherence among women was more clearly explained by demographic (i.e., marital status) and behavioral characteristics (i.e., alcohol, tobacco use). We should also note that the time to reported non-adherence among women was significantly shorter than that of men, thereby emphasizing the need for immediate action as ART treatment begins. Early intervention strategies to improve adherence should focus on these differences and on an integrated assessment of clinical, counseling, social, and work support, while facilitating access to health services. However, despite the evidence we have presented, additional studies are required to better ascertain the gender differences in non-adherence so that effective measures can be incorporated into the care of individuals living with HIV who use public services in Brazil. Despite the evidence provided by this analysis, further studies are still needed to address these gaps in knowledge and to better conceptualize gender differences in factors associated with non-adherence in AIDS public referral centers, with the ultimate aim of improving care and reducing the barriers to strong treatment adherence.
AUTHOR CONTRIBUTIONSGuimarães MD, Bonolo PF, Acúrcio FA and Ceccato MG conceived and designed the study. Campos LN and Rocha GM collected the data. Rocha GM performed the statistical analysis. Bonolo PF, Ceccato MG prepared the manuscript. All authors have read and approved the final version of the manuscript.
This study is part of the ATAR Project (Adherence to Antiretrovirals) developed by the Research Group in Epidemiology and Health Evaluation, Faculty of Medicine, Federal University of Minas Gerais, Belo Horizonte, MG, Brazil. Sponsorship: This research received financial support from the Pan-American Health Organization (OPAS/WHO) and the Brazilian National AIDS/STD/Hepatitis Department, UNESCO, Ministry of Health.
No potential conflict of interest was reported.