This retrospective study aimed to investigate the relationship between admission levels of serum γ-glutamyltransferase and poor myocardial perfusion after primary percutaneous coronary intervention in patients with acute myocardial infarction.
INTRODUCTION:Reperfusion injury caused by free radical release and increased oxidative stress is responsible for the pathophysiology of the no-reflow phenomenon in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Serum γ-glutamyltransferase is an established marker of increased oxidative stress.
METHODS:The study population consisted of 80 patients (64 men and 16 women, mean age = 67.5±6.6 years) with thrombolysis in myocardial infarction 0/1 flow pre-procedurally. The patients were divided into two groups according to thrombolysis in myocardial perfusion grades that were assessed immediately following primary percutaneous coronary intervention. The two groups (group 1 and group 2) each consisted of 40 patients with thrombolysis in myocardial perfusion grades 0-1 and thrombolysis in myocardial perfusion grades 2-3, respectively.
RESULTS:Admission pain to balloon time, γ-glutamyltransferase and creatine kinase-MB isoenzyme levels of group 1 patients were significantly higher than those of group 2 patients. Pain to balloon time, γ-glutamyltransferase, peak creatine kinase-MB isoenzyme, low left ventricular ejection fraction and poor pre-procedural thrombolysis in myocardial infarction grade were significantly associated with poor myocardial perfusion by univariate analysis. However, only pain to balloon time and γ-glutamyltransferase levels showed a significant independent association with poor myocardial perfusion by backward logistic regression analysis. Adjusted odds ratios were calculated as 4.92 for pain to balloon time and 1.13 for γ-glutamyltransferase.
CONCLUSION:High admission γ-glutamyltransferase levels are associated with poor myocardial perfusion in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention, particularly in patients with prolonged pain to balloon time.
The objective of primary percutaneous coronary intervention (pPCI) is to restore normal blood flow in the infarct-related artery (IRA). Previous studies have shown that preservation of the microcirculation is critical for a positive clinical outcome.1 It is well-known that achieving Thrombolysis in Myocardial Infarction (TIMI) grade 3 flow is insufficient to ensure myocardial salvage.1,2 In one-third of patients undergoing pPCI for acute myocardial infarction, echo-contrast ‘no-reflow' is observed after reperfusion therapy, despite TIMI grade 3 flow. This ‘no-reflow' phenomenon is associated with a higher incidence of congestive heart failure and left ventricular dysfunction.3 The TIMI myocardial perfusion grade (TMPG) was designed and validated to further risk-stratify patients in whom succsessful epicardial reperfusion was achieved.2 TMPG is a multivariate predictor of 30-day mortality, and is independent of age, gender, anterior MI location, admission pulse rate, corrected TIMI frame count, or TIMI flow grade.2 Previous clinical studies have clearly demonstrated that no-reflow predicts short- and long-term adverse clinical outcomes in patients presenting with acute myocardial infarction (AMI).4
Gamma-glutamyltransferase (GGT), an enzyme that is normally found in the serum as well as in the plasma membrane of virtually all cells except erythrocytes, catalyzes the first step in the degradation of extracellular glutathione (GSH). This degradation allows for precursor assimilation and reutilization of amino acids for synthesis of intracellular GSH, the primary thiol antioxidant in mammalian cells.5 Serum GGT level is widely used as a diagnostic test for hepatobiliary diseases and alcohol abuse.6 Several clinical studies have shown that elevated serum GGT is associated with all-cause mortality and increased risk of AMI.7 Additionally, degradation of GSH may play a pro-oxidant role under certain conditions. Low density lipoprotein (LDL) oxidation through GSH/GGT-dependent iron reduction has been suggested as an important mechanism in the pathogenesis of atherosclerosis.8 Furthermore, GGT activity has been observed in atherosclerotic coronary plaques.9 Reperfusion injury due to free radical release and increased oxidative stress has been suggested as a possible mechanism in the pathophysiology of the no-reflow phenomenon.10 Therefore, we aimed to examine the possible relationship between admission levels of GGT and myocardial perfusion grades after pPCI in patients presenting with AMI.
MATERIALS AND METHODSPatientsThe study population consisted of 40 patients with poor myocardial perfusion (TMPG 0-1) after pPCI and 40 age and sex-matched patients with better perfusion grades (TMPG 2-3) after the procedure (total 80 patients, 64 men and 16 women, mean age = 67.5±6.6 years). The retrospective selection included 265 acute ST-segment elevation myocardial infarction (STEMI) patients who were treated by pPCI from January 2003 to December 2007 at our institution. The diagnosis of AMI was established using ACC/AHA criteria.11 All patients presented TIMI 0/1 flow prior to intervention. The study population was divided into two groups according to TMPG assessed immediately after pPCI. No-reflow was defined as poor TMPG scores (TMPG 0-1) in the distal vasculature without apparent proximal coronary artery obstruction after intervention. Each group (group 1 and group 2) consisted of 40 patients with TMPG 0-1 and TMPG 2-3, respectively. Part of the data included in this study was previously published elsewhere.12
Patients with liver dysfunction and a history of chronic alcohol consumption, culprit lesion in the left main coronary artery, left main stenosis >50%, previous coronary artery bypass surgery, hemodialysis therapy, cardiogenic shock, pain to balloon time >12 hours, history of acute infection within the previous 10 days, presence of any chronic inflammatory-autoimmune disease, and any known malignancy were excluded from the study.
Coronary Intervention, Analysis of Collateral Circulation and TIMI Myocardial Perfusion GradeCoronary angiography (Siemens HICOR T.O.P Image System, Forcheim, Germany) was performed in multiple orthogonal projections using Judkins' technique. Coronary angiographic data were quantitatively analyzed. A stenosis of greater than 70% diameter in coronary arteries 1, 2, and 3 was defined as a one, two, or three-vessel disease, respectively. Collateral vessels were assessed according to the Rentrop classification.13 Routine stenting following balloon angioplasty was systematically attempted with standard techniques, including high-pressure balloon inflation (>14 atm) to expand deployed stents. A successful primary PCI procedure was defined as establishing a TIMI grade 3 flow in the artery responsible for AMI with a residual stenosis <20%.14 TMPG was graded densitometrically according to visual assessment of relative contrast opacification in the myocardial territory subtended by the infarct vessel in relation to epicardial density.1
EchocardiographyTransthoracic echocardiography was performed using an ESAOTE 2.5 MHz probe (ESAOTE, Genova, Italy) at the left lateral decubitis position before pPCI. Wall motion of 16 myocardial segments was interpreted according to the criteria of the American Society of Echocardiography.15
Blood ChemistryVenous blood samples were obtained on admission to the emergency room and analyzed for blood urea nitrogen, GGT, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatine kinase-MB isoenzyme (CKMB) levels. The next day, fasting plasma samples were obtained to measure: fasting plasma glucose (FPG), total serum cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol. Total serum cholesterol, TG, HDL, glucose, and GGT levels were measured via spectrophotometric technique on an Olympus AU-2700 autoanalyzer using commercial kits (Olympus, Hamburg, Germany). LDL cholesterol levels were calculated by the Friedwald formula.
Statistical AnalysisThe One Sample Kolmogorov-Smirnov and Levene tests were used to determine the distribution characteristics of variables and variance homogeneity. Results are expressed as mean ± SD, median (interquartile range) and percentages. The differences between groups were tested by chi-square, independent samples t-test and Mann-Whitney U tests. Differences were considered significant at p<0.05. We investigated the effects of different variables on myocardial perfusion grade by calculating the odds ratios in a univariate analysis for all variables. Variables displaying an unadjusted p-value <0.15 in logistic regression analysis were identified as potential risk markers and included in the multivariate regression analysis. We reduced the model using backward elimination, and we eliminated potential risk markers through likelihood ratio tests. Statistical analyses were performed using the SPSS 15.0 Statistical Package Program for Windows (SPSS Inc., Chicago, IL, USA).
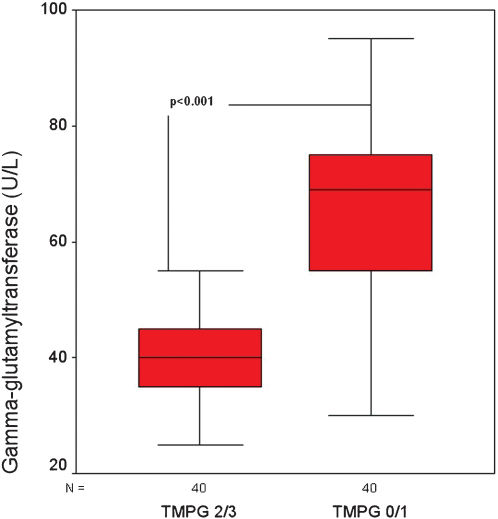
RESULTSThis retrospective study included 80 patients who presented with acute ST-segment elevation myocardial infarction (64 men and 16 women, mean age = 67.5±6.6 years). All patients presented TIMI 0/1 flow pre-procedurally. Post-procedural TMPGs were analyzed by two different interventional cardiologists who were blinded to the patients' clinical data. Intra- and interobserver variability for TMPGs 0-1 were 7.5% and 10.5%, respectively. Although intra- and interobserver variability for TMPG 2 were 1.9% and 2.7%, respectively, both the intra- and interobserver variability for TMPG 3 were 0%. In cases of disagreement between the observers, the cases were re-evaluated by both investigators and grouped according to the common final decision. Patients with TMPG 0-1 and 2-3 formed Group 1 (n = 40, 30 men, mean age = 68.8±5.3 years) and Group 2 (n = 40, 34 men, mean age = 66.2±7.5 years), respectively. Comparison between baseline demographical, clinical, and biochemical characteristics of patients with no-reflow and controls showed no statistically significant differences, except for peak CKMB and GGT levels, as shown in Tables 1 and 2 (for CKMB: 172.4±45.1 U/l vs. 144.5±37.8 U/l, p = 0.003 and for GGT: 64.32±16.43 U/L vs. 41.07±10.18 U/L, p<0.001; Figure 1). We performed an ROC curve analysis to define the optimal cut-off value of admission-GGT to predict no-reflow. A cut-off value of 50 U/L GGT predicted no-reflow with a sensitivity and specificity of 82.5% and 80%, respectively (AUC = 0.873; 95% CI = 0.791-0.955; p<0.001, Figure 2).
Baseline demographic and clinical characteristics of the study patients.
| Group 1 (n = 40) | Group 2 (n = 40) | p-value | ||
|---|---|---|---|---|
| Age (years) | 68.8±5.3 | 66.2±7.5 | 0.08 | |
| Sex (M), n (%) | 30 (75) | 34 (85) | 0.26 | |
| Age >70 years, n (%) | 14 (35) | 8 (20) | 0.13 | |
| BMI (kg/m2) | 25.9±2.0 | 25.5±1.7 | 0.42 | |
| Hypercholesterolemia, n (%) | 14 (35) | 12 (30) | 0.63 | |
| Diabetes mellitus, n (%) | 17 (42.5) | 15 (37.5) | 0.64 | |
| Family history, n (%) | 10 (25) | 6 (15) | 0.26 | |
| Hypertension, n (%) | 26 (65) | 21 (52.5) | 0.25 | |
| Smoking, n (%) | 19 (47.5) | 26 (65) | 0.12 | |
| Preinfarction angina, n (%) | 15 (37.5) | 17 (42.5) | 0.64 | |
| Previous MI, n (%) | 5 (12.5) | 7 (17.5) | 0.53 | |
| Admission HR (bpm) | 91±12 | 86±13 | 0.15 | |
| Killip Class | Class I, n (%) | 36 (90) | 37 (92.5) | 0.69 |
| Class II-III, n (%) | 4 (10) | 3 (7.5) | ||
| Preprocedural medications | Aspirin, n (%) | 15 (37.5) | 21 (52.5) | 0.17 |
| Nitrate, n (%) | 18 (45) | 15 (37.5) | 0.49 | |
| BAB, n (%) | 12 (30) | 16 (40) | 0.35 | |
| ACE-I, n (%) | 15 (37.5) | 19 (47.5) | 0.36 | |
| Statin, n (%) | 15 (37.5) | 22 (55) | 0.11 | |
I, Body mass index; MI, myocardial infarction; HR, heart rate; BAB, beta adrenergic blocker; ACE-I, angiotensin converting enzyme inhibitor.
Baseline biochemical characteristics of the study patients.
| Group 1 (n = 40) | Group 2 (n = 40) | p-value | |
|---|---|---|---|
| GGT (U/L) | 41.07±10.18 | 64.32±16.43 | <0.001 |
| Peak CKMB (U/l) | 172.4±45.1 | 144.5±37.8 | 0.003 |
| AST (U/L) | 50.90±9.68 | 52.81±12.27 | 0.44 |
| ALT (U/L) | 34.27±8.55 | 37.10±9.09 | 0.15 |
| Total cholesterol (mg/dl) | 253.75 (227-290) | 255.00 (236-300) | 0.37 |
| Triglyceride (mg/dl) | 165.00 (132-244) | 153.75 (144-254) | 0.85 |
| LDL cholesterol (mg/dl) | 172.87 (142-215) | 166.10 (142-224) | 0.55 |
| HDL cholesterol (mg/dl) | 40.50 (37-48) | 39.25 (27-50) | 0.57 |
GT, Gamma-glutamyltransferase; CKMB, Creatine kinase-MB isoenzyme; AST, Alanine aminotransferase; ALT, Aspartate aminotransferase; LDL, Low density lipoprotein; HDL, High density lipoprotein.
Results are expressed as mean±SD or median (interquartile range).
The two groups presented with similar angiographic, echocardiographic, and electrocardiographic characteristics. However, pain to balloon time and admission TIMI flow grades (TIMI flow 0-1) were significantly different between the two groups (Table 3). Direct stenting was performed in three patients from Group 1 and 2 patients from Group 2. Drug-eluting stents were used in two patients from each group. In Group 1, pain to balloon time was longer (6.07±0.72 h vs. 4.63±1.16 h, p<0.001) and the percentage of low admission TIMI flow grades was higher than for patients in Group 2 (95% vs. 80%, p = 0.03).
Angiographic, echocardiographic, and electrocardiographic characteristics of the patients.
| Group 1 (n = 40) | Group 2 (n = 40) | p-value | ||
|---|---|---|---|---|
| Pain to balloon time (h) | 6.07±0.72 | 4.63±1.16 | <0.001 | |
| Tirofiban use, n (%) | 13 (32.5) | 11 (27.5) | 0.62 | |
| Preprocedural TIMI 0/1, n (%) | 38 (95) | 32 (80) | 0.03 | |
| LVEF (<35%), n (%) | 16 (40) | 12 (30) | 0.34 | |
| Multivessel disease, n (%) | 13 (32.5) | 10 (25) | 0.45 | |
| Infarct- related artery | LAD, n (%) | 14 (35) | 16 (40.0) | 0.85 |
| RCA, n (%) | 20 (50) | 19 (47.5) | ||
| CFX, n (%) | 6 (15) | 5 (12.5) | ||
| Reference diameter (mm) | 3.03±0.26 | 3.12±0.23 | 0.20 | |
| Lesion length (mm) | 17.05±2.51 | 16.90±2.68 | 0.79 | |
| Final balloon pressure (Atm) | 15.25±2.06 | 15.82±1.78 | 0.18 | |
| Collateral score | 0.87±0.56 | 0.90±0.67 | 0.85 | |
| Number of Q waves | 2.92±1.24 | 2.40± 1.62 | 0.11 | |
| LVEF (<35%), n (%) | 16 (40) | 12 (30) | 0.34 | |
| WMS | 12.92±2.65 | 12.15±1.62 | 0.15 | |
LAD, Left anterior descending coronary artery; RCA, Right coronary artery; CFX, Circumflex coronary artery; MLD, Minimal lumen diameter; LVEF, Left ventricular ejection fraction; TIMI, Thrombolysis in myocardial infarction; WMS, Wall motion score.
The effects of different variables on myocardial perfusion were analyzed using univariate and multivariate logistic regression analyses. Data for the two groups was combined, and all the variables were analyzed via univariate analysis for predictors of poor myocardial perfusion. As shown in Table 3, pain to balloon time, GGT levels, peak CKMB levels, low LVEF, poor TIMI grade, wall motion score, and older age displayed a significant relationship with poor myocardial perfusion in the univariate analysis. When multivariate analyses were performed with eight variables in backward logistic regression analysis, only pain to balloon time and GGT levels continued to display a statistically significant independent association with poor myocardial perfusion in the model. Adjusted odds ratios were calculated as 4.92 for pain to balloon time (p = 0.009; CI = 1.50-16.13) and 1.13 for GGT (p<0.001; CI = 1.05-1.21), as shown in Table 4.
Effects of various variables on the myocardial perfusion grade assessed following primary percutaneous coronary intervention in univariate and multivariate logistic regression analyses.
| Unadjusted OR | 95% CI | p-value | Adjusted OR* | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Age >70 years | 2.15 | 0.78–5.92 | 0.13 | 1.79 | 0.23-14.05 | 0.57 | |
| WMS | 1.14 | 0.94-1.39 | 0.16 | 1.01 | 0.65-1.58 | 0.93 | |
| Number of Q waves | 1.29 | 0.94-1.77 | 0.11 | 1.15 | 0.57-2.31 | 0.69 | |
| Peak CKMB | 1.10 | 1.00-1.02 | 0.009 | 1.00 | 0.98-1.02 | 0.44 | |
| LVEF (<35%) | 3.11 | 1.18-8.20 | 0.02 | 3.44 | 0.50-23.38 | 0.57 | |
| TIMI 0/1 | 4.05 | 1.56-10.51 | 0.004 | 2.90 | 0.47-17.77 | 0.24 | |
| Pain to balloon time | 6.36 | 2.70-14.95 | <0.001 | 4.92 | 1.50-16.13 | 0.009 | |
| GGT (U/L) | 1.15 | 1.09-1.22 | <0.001 | 1.13 | 1.05-1.21 | 0.001 | |
| Diabetes mellitus | 1.23 | 0.50-3.01 | 0.64 | ||||
| Killip | Class I | 1.00 | |||||
| Class II-III | 1.37 | 0.28-6.55 | 0.69 | ||||
| Tirofiban use | 0.78 | 0.30-2.05 | 0.62 | ||||
| Multivessel disease | 1.44 | 0.54-3.82 | 0.46 | ||||
| Reference diameter | 0.30 | 0.05-1.87 | 0.20 | ||||
| Lesion length | 1.02 | 0.86-1.21 | 0.79 | ||||
| Final inflation pressure | 0.85 | 0.67-1.07 | 0.18 | ||||
| Collateral score | 0.93 | 0.45-1.91 | 0.85 | ||||
WMS, Wall motion score; CKMB, Creatine kinase MB isoenzyme; LVEF, left ventricular ejection fraction; TIMI, Thrombolysis in myocardial infarction; GGT, Gamma-glutamyltransferase.
Our study revealed that in patients with AMI undergoing pPCI, high admission GGT levels are associated with lower myocardial perfusion grades after intervention.
The no-reflow phenomenon is primarily attributed to the disturbed microvascular integrity that occurs due to free radical release, microvascular constriction and obstruction, platelet microembolism, thrombosis and neutrophil aggregation and plugging.16–18
Reperfusion of the ischemic myocardium in AMI results in oxidative stress caused by the release of quantities of reactive oxygen species (ROS) that exceed the neutralizing potential of the cells' anti-oxidative defense mechanisms.19 Oxidative cellular damage induced by high levels of reactive oxygen species is considered a primary cause of lethal myocardial reperfusion injury. The generation of reactive oxygen species during reperfusion is a complex process involving cardiomyocytes, endothelial cells, and activated neutrophils. Re-energization of the electron transport chain in the mitochondria of cardiomyocytes leads to the formation of ubiquinone and oxygen-derived radicals.20,21 After breaking down ATP, post-ischemic cardiomyocytes release large amounts of adenosine and inosine, which are further degraded by xanthine oxidase in endothelial cells, constituting another important source of ROS.21
Serum GGT level has been suggested as an independent cardiovascular risk factor.22 It is also a predictor of all-cause and coronary heart disease-related mortality, independent of alcohol intake or liver disease.7 GGT expression is increased by oxidants, and GGT activity serves as a marker for oxidative stress in rat lung epithelial cells.23 Increased serum GGT activity can be used as a marker for increased oxidative stress in humans.24 Increased GGT activity is also present in atherosclerotic coronary plaques.8
Oxidative and inflammatory events are closely related in the pathophysiology of acute coronary syndrome (ACS).25 Serum GGT activity has been suggested as an independent predictor of major adverse cardiovascular events in patients with ACS during coronary care unit stay and after hospitalization.26
In the microvasculature of the heart, post-ischemic reperfusion results in endothelial cell swelling and luminal membrane blebbing, both of which may contribute to the no-reflow phenomenon.27 Ischemia results in impaired antioxidant defense, and subsequent reperfusion results in an increased concentration of reactive oxygen species.19 In the current study, we demonstrated that increased pre-procedural GGT levels display a statistically significant independent association with poor myocardial perfusion. From this perspective, we suggest that increased pre-procedural oxidative stress represented by GGT levels may be an important factor contributing to microvascular damage in patients with AMI. Higher levels of GGT in patients with poor TMPG may be the result of increased microvascular injury and increased oxidative stress. High GGT activity can also represent a compensatory response against the microvascular injury caused by free radicals. It is also reasonable to expect increased GGT activity due to delayed pain-balloon time and subsequent increased myocardial injury. On the other hand, peak CKMB levels, which are a specific marker of myocardial injury, have been found to be independent of TMPG. Therefore, the significant association between TMPG and increased GGT activity as a non-specific marker for myocardial injury highlights the importance of oxidative stress in reperfusion injury, poor TMPG and ischemic injury.
The role of admission GGT levels in the prediction of poor myocardial perfusion grades after pPCI in patients with AMI has not been extensively studied.28 The dynamic nature of acute coronary syndromes is usually associated with spontaneous ischemia-reperfusion injury in the infarct-related artery.29 Therefore, we considered the fact that poor myocardial perfusion after pPCI is not only related to procedural factors and clinical characteristics of the patients but may also be related to reperfusion injury and oxidative stress occurring before coronary intervention. GGT can be used as a marker of poor post-procedural flow. However, the current data is not sufficient to suggest GGT as a causal agent.
The major limitations of this study are small sample size and retrospective design. Additionally, self-reported alcohol consumption may not be reliable. This study did not include a control group to compare the GGT levels of the AMI patients with healthy subjects. Lastly, we did not analyze other important plasma markers of oxidative stress, such as oxidized LDL and plasma and/or intracellular GSH levels.
In conclusion, high admission GGT levels are associated with poor myocardial perfusion after pPCI in patients with AMI. High GGT levels and door to balloon time relate to the degree of reperfusion after pPCI. An animal model comparing reperfusion injury in animals with higher and lower GGT levels would provide further evidence to support our findings. Additional large-scale randomized studies are needed to clarify the clinical utility of admission GGT levels in the prediction of poor myocardial perfusion.
No potential conflict of interest was reported.