Critical illness has a major impact on the nutritional status of both children and adults. A retrospective study was conducted to evaluate the incidence of hospital malnutrition at a pediatric tertiary intensive care unit (PICU). Serum concentrations of IL-6 in subgroups of well-nourished and malnourished patients were also evaluated in an attempt to identify those with a potential nutritional risk.
METHODSA total of 1077 patients were enrolled. Nutritional status was evaluated by Z-score (weight for age). We compared mortality, sepsis incidence, and length of hospital stay for nourished and malnourished patients. We had a subgroup of 15 patients with severe malnutrition (MN) and another with 14 well-nourished patients (WN). Cytokine IL-6 determinations were performed by enzyme-linked immunosorbent assay.
RESULTS53% of patients were classified with moderate or severe malnutrition. Similar amounts of C- reactive protein (CRP) were observed in WN and MN patients. Both groups were able to increase IL-6 concentrations in response to inflammatory systemic response and the levels followed a similar evolution during the study. However, the mean values of serum IL-6 were significantly different between WN and MN patients across time, throughout the study (p = 0.043).
DISCUSSIONa considerable proportion of malnourished patients need specialized nutritional therapy during an intensive care unit (ICU) stay. Malnutrition in children remains largely unrecognized by healthcare workers on admission.
CONCLUSIONSThe incidence of malnutrition was very high. Malnourished patients maintain the capacity to release inflammatory markers such as CRP and IL-6, which can be considered favorable for combating infections On the other hand, this capacity might also have a significant impact on nutritional status during hospitalization.
Systemic inflammatory response causes metabolic deregulation leading to muscle proteolysis (hypercatabolism)1 and the release of cytokines and chemokines in critically ill patients. The resulting negative nitrogen balance is usually proportional to the intensity of the injury2. Interleukin 6 (IL-6), a proinflammatory cytokine recognized as an early marker of the Systemic Inflammatory Response Syndrome (SIRS) in several disease models, might be used to determine whether the inflammatory response is intact.3,4 More recently, serum concentrations of IL-6 have been associated with the capacity to identify patients with a potential nutritional risk.1,3,4
Although hospital malnutrition constitutes an important risk factor for increases in morbidity, lethality, length of hospital stay, and medical costs, it is troublesome to assess the impact of acute hospitalization on the nutritional status of critically ill children.5,6 Associations have been reported between poor nutritional status, decreased respiratory function, impaired wound healing, and immune and gastrointestinal dysfunctions.4,7,8
The diagnosis of malnutrition is generally based on objective measurements of nutritional status, including assessments of oral intake, weight loss, anthropometric data, determination of cell-mediated immunity, biochemical parameters, and body composition analysis.6,9 Despite its high prevalence, malnutrition is seldom recognized by pediatricians in hospitalized patients. As a consequence, nutritional therapy is underprescribed, thus compounding the problem. Therefore, assessment of the nutritional status of patients is important as a guide to early nutritional intervention.10–12
Malnutrition is common at hospital admission and tends to worsen during hospitalization. In Europe and North America, 40–50% of hospitalized patients are at risk of malnutrition. Several studies performed in Brazil and in other countries demonstrated that malnutrition can affect 50% of children and adolescents during hospitalization, although the classical kwashiorkor presentation is rare in critically ill infants, children, and adolescents with chronic diseases such as those in our ICU. In general, little is known about the nutritional status of critically ill children during hospitalization in, and after discharge from, ICUs. Critical illness has a major impact on the nutritional status of both children and adults. Studies conducted more than 20 years ago had already demonstrated that 15–20% of children admitted to pediatric intensive care units were acutely or chronically malnourished.10–14
We conducted a retrospective study to evaluate the incidence of hospital malnutrition in children and adolescents during the first 72 hours after admission in our tertiary intensive care unit. We also evaluated serum concentrations of IL-6 and C- reactive protein (CRP) in groups of well-nourished and malnourished patients, in an attempt to determine those with a potential nutritional risk.
PATIENTS AND METHODSThe study was approved by the Institutional Research Ethics Committee. A total of 1077 children and adolescents admitted from March, 2002 to February, 2005 to our level III pediatric ICU were enrolled. We classified the nutritional status by Z-score weight for age in all patients admitted during this period. We considered a Z score of (−2) or lower as moderate or severe malnutrition. We compared mortality, sepsis incidence, and length of hospital stay for nourished and malnourished patients. The patient data were recorded, and Z scores were calculated for body weight/age using the EPI 2000 program from the Centers for Disease Control and Prevention (Atlanta, GA., USA). This software uses the National Center for Health Statistics 2000 (NCHS) reference values. We used a nonparametric statistic analysis (Kruskal-Wallis test) to test the data.
Twenty-nine critically ill children and adolescents aged between two months and 16 years were included in two groups selected from the total population of 1077 children. We had a group of 15 patients with severe malnutrition (MN) and another with 14 well-nourished patients (WN), all of 29 presenting with SIRS according to a consensus conference on pediatric sepsis15. The two subgroups were, for convenience, recruited by sequential sampling according to the order of hospitalization during the period of study. Severity of illness was measured at admission by the Pediatric Risk of Mortality score16 (PRISM) and the Therapeutic Intervention Scoring System17 (TISS, repeated on the fifth and tenth days). The nutritional evaluation was comprised of: Z scores calculated for body weight/age and weight/length, upper arm muscle circumference, serum C-reactive protein (CRP) and albumin. Interleukin 6 serum levels, representing the magnitude of inflammatory response, were compared between MN and WN on the first, fifth, and tenth days. All patients received early parenteral and/or enteral nutrition with standardized calorie and protein levels. Newborn babies, patients with leucopenia (total leucocyte count < 1000 mm3), severe liver dysfunction or who received corticosteroids for more than 72 hours were excluded.
Serum cytokine IL-6 determinations were performed by means of an enzyme-linked immunosorbent assay, according to the manufacturer instructions (R&D Systems, Abington, UK). Each sample was tested in triplicate, and the mean value was obtained. The IL-6 detection limit was 0.7 pg/mL. Cytokine concentrations in malnourished and well-nourished patients were compared using two-way ANOVA for repeated measures.
RESULTSOf the total population (1077 patients), 53% were classified with moderate or severe malnutrition. When only infants were analyzed, the incidence of moderate and severe malnutrition was even higher (58%). The general mortality rate was 18.5%. Table 1 shows the mortality rate, sepsis incidence, and period of hospitalization in the ICU in well-nourished, moderately malnourished [Z score between (−1) and (−2)], and severely malnourished patients.
Mortality rate, sepsis incidence and the period of hospitalization at the ICU in well-nourished, moderate malnourished and severe malnourished patients
| Well-nourished | Moderate malnourished | Severe malnourished | |
|---|---|---|---|
| Mortality rate | 18.5% | 19.5% | 17.9% |
| Sepsis incidence | 34% | 39% | 36% |
| Period of hospitalization at the ICU | 5 days | 5 days | 8 days |
n= 1077 patients;
*There was not significant difference when the groups were compared.
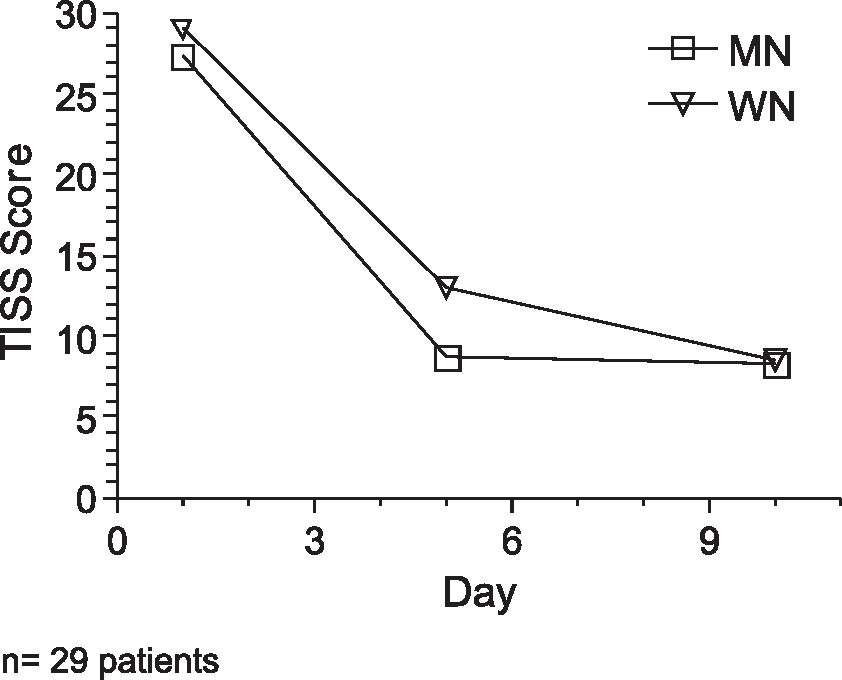
When we analyzed the two groups (MN and WN), containing 29 patients, there were no significant gender differences. Table 2 summarizes the subgroup characteristics. PRISM and TISS clinical scores indicated similar severity of illness in both groups throughout the study (Figure 1). The median ages of malnourished and well-nourished patients were 25.6 months and 10.7 months, respectively. One well- nourished and four malnourished patients died during the evaluation.
Description of the diagnosis of the 29 selected patients according to age
| Malnourished (Mn) | Well-nourished (Wn) |
|---|---|
| Median of age months = 25.6 | Median of age in months = 10.7 |
| NCS tumor (pneumonia and sepsis) | Seizures (ARDS and sepsis) |
| Cardiac malformation (sepsis) | Bilateral pneumonia (sepsis) |
| Pneumonia (sepsis) | Atopic Eczema (sepsis) |
| Myocarditis (pneumonia and sepsis) | Seizures (peumonia and sepsis) |
| Leukemia (sepsis) | Pleural empyema (sepsis) |
| Cutaneous Leishmaniosis (sepsis) | Mandibular abscess (sepsis) |
| Short Bowel Syndrome (sepsis) | Pleural empyema (sepsis) |
| Pulmonary hypertension (pneumonia and sepsis) | Pneumonia (sepsis) |
| Peritonitis (sepsis) | Pneumonia (sepsis) |
| Megacolon (sepsis) | Renal malformation (sepsis) |
| Encephalopathy (gastroenteritis and sepsis) | Bronchopleural fístula (sepsis) |
| Meningitis (sepsis) | Cardiac dysfunction (pneumonia and sepsis) |
| Neonatal asphyxia (pneumonia and sepsis) | Pneumonia (sepsis) |
| Pneumonia and sepsis | Pleural empyema (sepsis) |
| Renal infection (sepsis) |
Malnourished patients had lower mean (± SD) Z scores for weight/age (−3.1 ± 2.0) and for weight/length (−1.4 ± 2.3) on the first day, as compared to the mean Z scores for weight/age (0.2 ± 0.9) and weight/length (1.1 ± 1.5) in well-nourished children and adolescents. Z scores of weight/age worsened (p < 0.05) only in well-nourished patients during the period of the study (Table 3). Table 4 shows the evolution of results for upper arm muscle circumference, CRP, and albumin. Similar amounts of CRP were observed in WN and MN patients. There were no albumin concentration differences during the study in either group. There were no significant differences between the groups with respect to the upper arm muscle circumference. However, there were significant decrements in other well-nourished group parameters during the study period.
Z score for weight/age and weight/length in malnourished and well-nourished patients on the first, fifth and tenth days
| Day | Weight/ age | Weight/ length | |||
|---|---|---|---|---|---|
| Well-nourished | Malnourished | Well-nourished | Malnourished | ||
| 1th | Mean±SD | 0.2±0.9 | −3.1±2.0 | 1.1±1.5 | −1.4±2.3 |
| 5th | Mean±SD | −0.3±1.1 | −3.1±2.0 | 0.1±2.0 | −1.8±2.4 |
| 10th | Mean±SD | −0.5±1.1 | −2.5±1.7 | 0.3±2.0 | −1.6±3.3 |
n= 29 patients
Means values of upper arm muscle circumference, concentrations of albumin and C-reactive protein in malnourished and well-nourished patients during the study
| Day | Malnourished | Well-nourished | |||||
|---|---|---|---|---|---|---|---|
| Upper arm muscle circumference (cm) | Albumin (g/dL) | C-Reactive protein (mg/L) | Mid upper arm circumference (cm) | Albumin (g/dL) | C-Reactive protein (mg/L) | ||
| 1th | Mean±SD | 10.6±2.8 | 3.1±0.8 | 129.9±124.6* | 10.7±1.1** | 2.8± 0.7 | 110.5±115.6*** |
| 5th | Mean±SD | 9.9±3.4 | 3.4±0.9 | 54.4±99.2 | 9.7±1.8 | 3.3± 0.7 | 45.5± 45.2 |
| 10th | Mean±SD | 10.4±3.7 | 3.3±0.9 | 54.9±79.6* | 9.1±1.6** | 3.3±0.7 | 25.8± 30.0*** |
n= 29 patients;
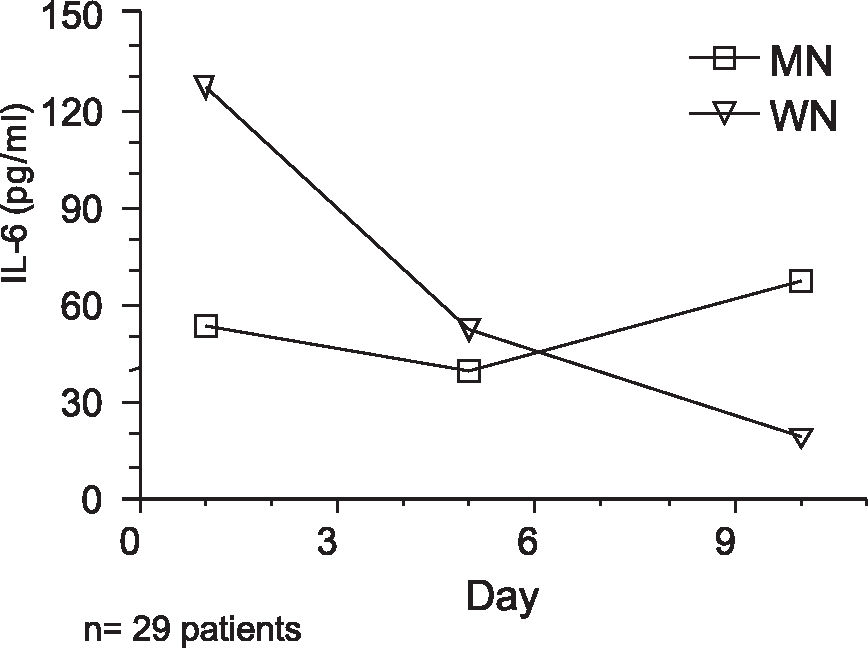
Age had no influence on IL-6 levels. Both groups were able to increase IL-6 concentrations and levels underwent a similar evolution during the study. However, the mean values of serum IL-6 were significantly different between Wn and Mn patients across time (p = 0.043). Tables 5 and 6 and Figure 2 show the results with repeated mean measures.
Descriptive statistics with estimated marginal means of IL-6
| Day | Malnourished | Well-nourished | ||||
|---|---|---|---|---|---|---|
| 1th | 5th | 10th | 1th | 5th | 10th | |
| Mean±SD | 53.73± 63.5 | 39.32±50.9 | 67.38±141.0 | 127.21±181.3 | 52.60±43.3 | 18.81±22.6 |
n= 29 patients;
*SD = standard deviation; Considered normal serum levels = 3.5 pg/mL; R&D systems, UK
In a study following pediatric critical care children, Hulst et al. described in 2004 that these patients had a poor nutritional status very frequently compared to that of the general population. In their study, 24% of children were acutely and/or chronically undernourished. The period of illness prior to admission and/or the high prevalence of underlying disease in these children (84%) could explain this high proportion. They concluded that a considerable proportion of malnourished patients need specialized nutritional therapy during ICU stays9. These characteristics were very similar to those found in the present study, with a high prevalence of malnutrition in children and adolescents admitted to our tertiary ICU. Öztürk et al. reported that, in well-nourished children, there was no deterioration of their anthropometric values during hospitalization. However, there was also no improvement in anthropometric values of initially malnourished children in the 1–24-month age group. They concluded that malnutrition on admission in children often remains unrecognized by healthcare workers10. As already mentioned, the classical kwashiorkor presentation is rare in children and adolescents with chronic diseases1. Our results corroborated these data: in our study groups, we did not find any patients with significant nutritional edema.
Scrimshaw documented the synergistic relationship between malnutrition and infection, representing at the same time the cause and the consequence of an acquired immune deficiency; these phenomena have been amply described since the 1970s.18 Many studies have described the prevalence of disease-related malnutrition in hospitalized patients.1,5,11,12 Others emphasized the association of malnutrition with discharge, prolonged hospitalization, and the occurrence of pneumonia and weight loss during a hospital stay.11 It is possible that doctors and nurses are still unaware of the importance of performing the screening and prompt treatment of malnutrition. It is also likely that malnourished patients might present with a variety of atypical symptoms that remain undetected, preventing the diagnosis of malnutrition.10,19 Critical illness has a major impact on the nutritional status of both children and adults.2 In the present study, the incidence of malnutrition was very high, and moderate or severe malnutrition can provoke a poorer prognosis and a higher mortality odds ratio.19 When we analyzed a group of WN and MN patients, we found similar results concerning the severity of disease as demonstrated by TISS and PRISM scores. Nevertheless, it is difficult to establish early indicators of a prolonged intensive care unit stay or the impact of malnutrition on mortality rates. Other factors, such as the severity of disease, early need for - and sustained dependence on - mechanical ventilation, inadequate sedation, coma, and type of infection, might also influence mortality rates and hospital stay.19,20
Serum IL-6 levels appear to be an ideal marker for detecting early-onset neonatal infection with a high degree of sensitivity and specificity.3 Studies have demonstrated that the acute response in severely malnourished children is achieved either through a decrease in catabolism or a combination of increased synthesis and decreased catabolism.21–23 It has been reported that albumin alone can underestimate the nutritional risk, while IL-6 combined with C-reactive protein has been suggested for identifying infected infants in the postnatal period. IL-8 and calcitonin precursors have been used to diagnose infections in neutropenic children.3,23–25 In our study, IL-6 concentrations correlated with the severity of illness determined by PRISM and TISS on the first day of hospitalization.
The chronic inflammatory response in patients with severe malnutrition is a controversial subject. Some studies suggest a decrease in inflammatory markers in malnourished patients, while others suggest similar intensity of inflammation compared with well-nourished children. Other reports suggest deregulated cytokine production, with a complex interplay of elevated pro- and anti-inflammatory mediators.25,26 In the present study, both Wn and Mn groups had similar levels of IL-6 on the first and fifth days after hospitalization.
Sepsis is still a major and frequent cause of morbidity and death in ICUs, where early diagnosis and treatment are critical to improved prognoses. The incidence of sepsis was very high in the present study, and serum concentrations of IL-6 were increased in both groups throughout the study (until the 10th day). MN patients had a preserved capacity to increase CRP synthesis. The main effects of this protein are: opsonizing capacity, promotion of chemotaxis and phagocytosis, and stimulation of lymphocyte proliferation and cytotoxicity. Increased acute-phase proteins are of clinical relevance because they may have roles in combating infections. There was a significant decrease of CRP in both MN and WN children throughout the study. These CRP results, together with the preserved IL-6 production, suggest that malnourished patients retain the capacity to mount a positive acute-phase response during infection.1,25,26
CONCLUSIONSThe frequency of malnutrition among pediatric ICU patients is very high. Malnutrition is difficult to recognize in a nonspecific hospital population. To be able to correctly identify all malnourished patients, screening of the nutritional status of all newly admitted patients is imperative. Malnutrition may also have contributed to disease severity. Malnourished patients preserve the capacity to release inflammatory markers such as CRP and IL-6, which can be considered favorable for combating infections. Conversely, this release might also have a significant impact on nutritional status during hospitalization.