The association between diabetes and Strongyloides infection remains controversial. This study aimed to detect Strongyloides stercoralis DNA in the feces of patients with Diabetes Mellitus type 2 (DM2).
MethodsFecal samples were analyzed via the Lutz, Rugai, and agar plate culture methods. PCR amplification was performed using two targets (PCR-genus and PCR-species) located on the S. stercoralis 18S ribosomal.
ResultsThe positivity for S. stercoralis using parasitological methods was 1.1%. PCR-genus (14.13%) demonstrated a higher positivity than PCR-species (9.78%).
ConclusionThe results confirm the greater positivity of the molecular diagnosis in relation to parasitological methods, reinforcing its use as an additional tool for the diagnosis of S. stercoralis infection in patients with DM2 living in endemic areas for this helminthiasis.
Strongyloides stercoralis infection is a neglected tropical disease1 that affects approximately 350 million people worldwide, particularly in tropical and subtropical regions.2,3 The ability of S. stercoralis to cause systemic infection is an important feature of this parasite that may lead to hyperinfection syndrome and disseminated strongyloidiasis with a mortality rate of up to 100%, especially in the presence of immunological impairment.4,5
Diabetes mellitus is a metabolic disease and a serious health problem. Type 2 Diabetes Mellitus (DM2) is the most common form in terms of the number of people affected, disability, and premature mortality.6 Evidence suggests that inadequate control of blood glucose levels in diabetic patients contributes to susceptibility to infections,7,8 including parasitic infections.9 However, the relationship between diabetes and Strongyloides infection remains controversial, with both positive10 and negative11 associations. Furthermore, the presence of clinical situations associated with immunosuppression, such as prolonged use of corticosteroids, can predispose individuals to the development of severe forms of S. stercoralis infections.12-15
The definitive laboratory diagnosis of S. stercoralis infection is based on the detection of larvae in the feces by microscopy. However, confirmation of infection is difficult because of the small number of larvae released in one's feces, particularly in the case of chronic infections.5 Molecular diagnosis is considered highly sensitive compared to parasitological methods and has been used to detect S. stercoralis infection in stool samples.16,17 In the context of S. stercoralis infection and diabetes, to date, very little research attention has focused on PCR for specific DNA detection.15,18 Thus, the present study aimed to detect S. stercoralis DNA in the feces of patients with DM2.
MethodsEthics statementThis study was approved by the Research Ethics Committee of the Federal University of Goiás, GO (protocol number 929.187/2015) and by Secretaria Municipal de Saúde de Jataí, GO. Informed consent was obtained from each patient before specimen collection.
Study populationThis study was conducted in the municipality of Jataí, Brazil, which is located in southwestern Goiás State, 327 km from Goiânia (the capital of Goiás State) and 535 km from Brasilia (the capital of Brazil). The Health Care Network has 16 family health teams, corresponding to a population coverage of approximately 61.4%. The municipality of Jataí has an estimated population of 102,065 inhabitants.
The study was conducted from January 2015 to December 2016 and included patients with DM2 from the Diabetes Education and Control Program treated at the basic health unit of the municipality of Jataí, Goiás State. Inclusion criteria included any sex, age ≥30 years, diagnosis of DM2, use of insulin > 5 years, blood tests within the last two years, glycated Hemoglobin (HbA1c) > 6.5%, and no use of anthelmintic drugs in the last six months. Sociodemographic, clinical, and laboratory data were analyzed. All results were reported to the patients.
Parasitological diagnosticThree fresh fecal samples were collected on alternate days from each individual and sent to the Laboratório de Parasitologia, Universidade Federal de Jataí, Goiás for processing and analysis. The samples were analyzed using the Lutz, Rugai, and agar plate culture methods. Aliquots of samples were immediately frozen at -20°C for molecular analysis.
Molecular diagnosticDNA extractionThe molecular analysis was carried out at Laboratório de Investigação Médica (LIM06) at the Hospital das Clínicas of the Universidade de São Paulo, São Paulo, Brazil. DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen Hilden, Germany) according to the manufacturer's modified instructions. A pool of three fecal samples from each patient was prepared (∼600 mg), followed by washing with a 2% Polyvinylpolypyrrolidone (PVPP) solution (Sigma-Aldrich, San Luis, Missouri, USA) in phosphate buffer (0 0.01 M, Ph 7.2). The pellet was used for DNA extraction. The DNA was then quantified using a NanoDrop ND-100 UV-VIS V3.2.1 (NanoDrop Technologies, Wilmington, DE, USA).
Polymerase Chain Reaction (PCR)PCR amplification was performed using two sets of primers located on the S. stercoralis 18S ribosomal: genus-specific (PCR-genus [392bp, forward 5′-AAAGATTAAGCCATGCATG-3′ and reverse 5′-GCCTGCTGCCTTCCTTGGA-3′])19 and species-specific (PCR-species [101bp, forward 5’-GAATTCCAAGTAAACGTAAGTCATTAGC-3’ and reverse 5′-TGCCTCTGGATATTGCTCAGTTC-3]).17
The PCR reaction tests were performed at a volume of 25 μL containing ∼50 ng μL−1 of DNA, 2.0 μg of BSA, 0.2 mM each of dNTP, 1.5 mM MgCl2, 2 pM of each primer, a 1 × PCR buffer, and 0.5 U Platinum® Taq DNA polymerase (Invitrogen™, Thermo Fisher Scientific Corporation, Waltham, MA, USA). Amplification cycles were composed of an initial denaturation at 94°C for 2 min, followed by 30 cycles of 94°C for 1 min (denaturation), 60°C for 1 min (annealing), 72°C for 1 min (extension), and 72°C for 2 min (final extension). PCR amplification was conducted using a Mastercycler EP Gradient S Thermocycler (Eppendorf, Hamburg, Germany). The products were separated by electrophoresis in 2% agarose gel containing SYBR Safe (Invitrogen™). Negative (PCR mix with no DNA template) and positive (DNA from the filariform larvae of S. stercoralis collected from positive agar plates) controls were included in each amplification run. PCR products with positive amplification for each target were submitted for sequencing, and the sequences obtained were evaluated using the Basic Local Alignment Search Tool (BLAST).
Data analysisDescriptive analyses, including mean, Standard Seviation (SD), and percentages, were used to analyze the data.
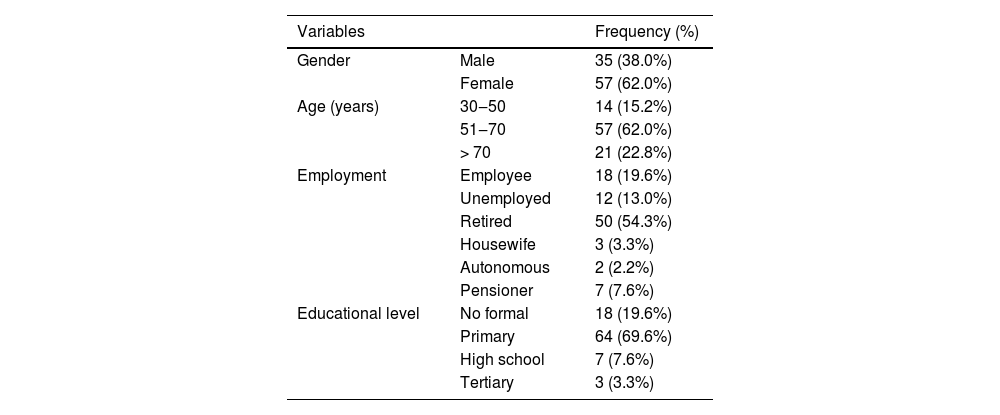
ResultsSociodemographic data and parasitological diagnosisA total of 92 patients with DM2 with a mean age of 62.3 years (±10.4) were included. Of these, 57 (61.96%) were women and 35 (38.04%) were men. Most of the patients were retired and had completed elementary school (Table 1).
Socio-demographic (gender, age, employment, and education level) data of diabetes mellitus type 2 patients included in the study (n = 92).
Based on the parasitological results, positivity in association with DM2 was 32.61% (30/92) for parasites and intestinal commensals. Regarding helminths, only one case (1.1%) of S. stercoralis associated with DM2 was observed. In addition, the protozoans Blastocystis sp. (7.61%), Entamoeba coli (5.43%), Entamoeba hartmanni (4.35%), Endolimax nana (3.26%), Entamoeba histolytica (2.17%), and Giardia lamblia (1.1%) were observed.
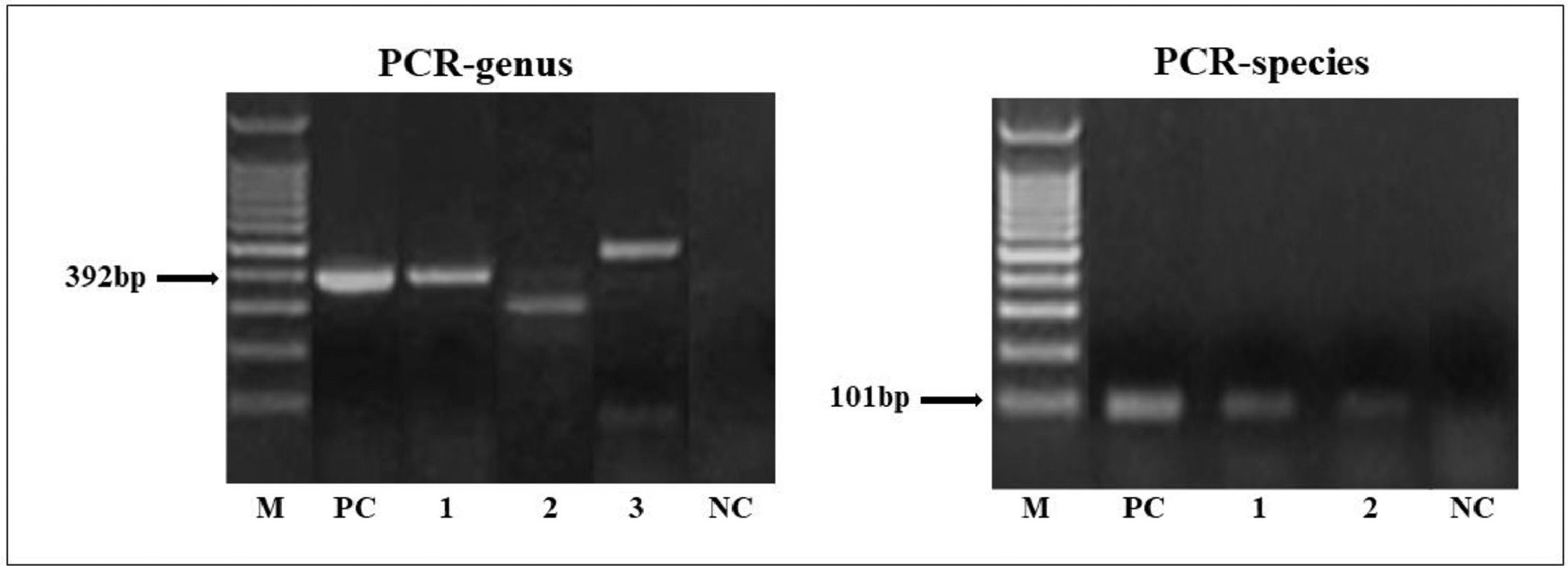
Molecular diagnostic of the Strongyloides stercoralis infectionIn the PCR-genus, target fragment amplification (∼392 bp) was observed in 14.13% (13/92) of patients with DM2 (Fig. 1). The sequences obtained from the PCR genus were of low quality. It is important to note that of the eight patients with positive results in the PCR-genus test, four showed parasitological positivity for Blastocystis sp./E. hartmanni or E. nana, and three were positive for E. nana or/and E. coli. Non-specific amplification (∼400‒500 bp) were observed in 18 samples. Among these, Blastocystis sp. and amoebas were identified in six samples by parasitological methods.
Strongyloides DNA amplification by PCR-genus (392 bp) and PCR-species (101 bp). M, 100 bp Molecular weight marker, NC, Negative Control (PCR mix with water); PC, Positive Controls (S. stercoralis DNA larvae), and DNA of fecal samples from diabetes mellitus type 2 (lane 1, 2 or 3).
The PCR species showed a positivity rate of 9.78% (9/92) in patients with DM2 (Fig. 1). All PCR-species products were of high quality and confirmed the identity of S. stercoralis. Six samples with positive amplification by PCR-species (∼101 bp) showed specific amplification by PCR-genus (∼392 bp). The patients with parasitological results that were positive for S. stercoralis were positive only in the PCR-species test.
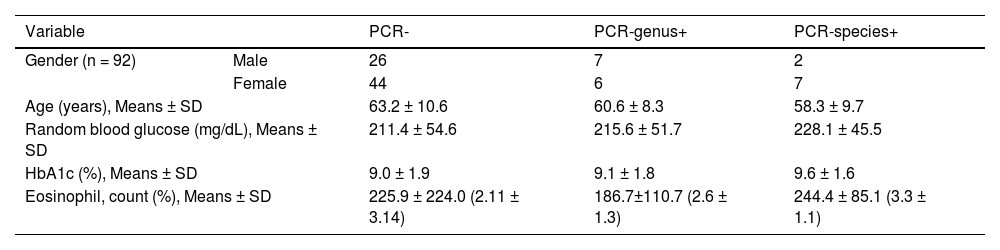
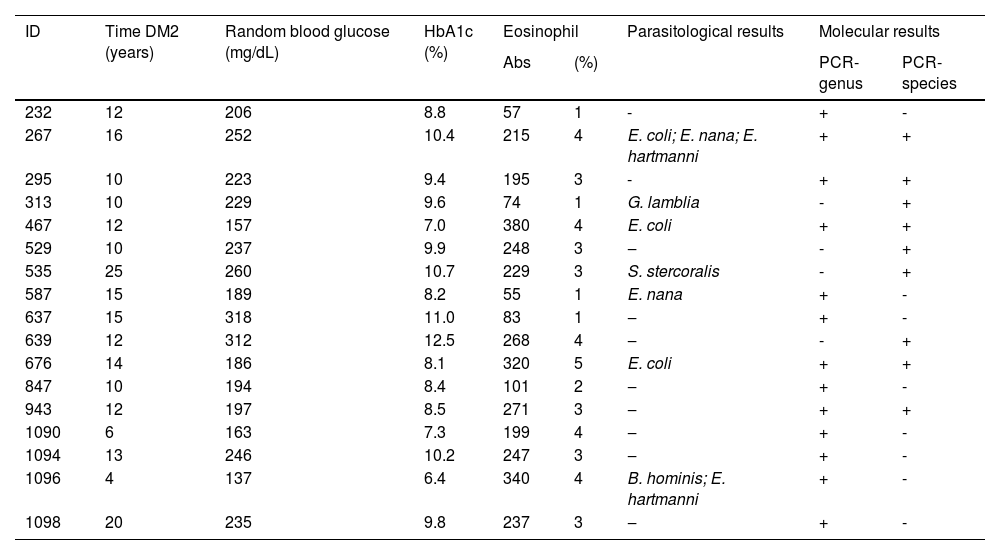
The mean values of glycemia (228.1 mg/dL), HbA1c (9.6%), and eosinophils (244.4% and 3.3%) were higher in the PCR-species tests for DM2-positive patients (Table 2). Patients with positive amplification results are shown in Table 3. The mean HbA1c level was 9.2%, and the time since diabetes diagnosis ranged from 4 to 25 years (mean 12.7).
Socio-demographic (gender, age) and laboratory (random blood glucose, HbA1c, eosinophil) data of diabetes mellitus type 2 patients (n = 92) according to the molecular results.
Characterization (Time DM2, random blood glucose, HbA1c, eosinophil, parasitological results) of diabetes mellitus type 2 patients according to the molecular results.
Despite decades of investigation, the association between diabetes and Strongyloides infection remains controversial.10,11,20-22 Notably, strongyloidiasis is a neglected tropical disease and detection tests used in primary health care generally have low sensitivity.23 In addition, severe forms of strongyloidiasis have been reported in patients with diabetes, particularly when they have a condition associated with immunosuppression.13,14
Several parasitological techniques have been used for the diagnosis of S. stercoralis infection;4,5 however, the positivity rate is usually low, which can lead to false-negative results. In the present study, a 1.03% positivity rate for S. stercoralis was detected using parasitological techniques. Similar results were reported in a study of parasite frequencies in individuals with type 1 and type 2 diabetes in the Federal District of Brazil.24 In a review study,25 Brazil was characterized as a hyperendemic area, with an occurrence of 5.5% for S. stercoralis infection and an estimated frequency of 6.6% in the Midwest region.
Analysis of three stool samples per individual via the Rugai and agar plate culture methods ‒ techniques indicated for the search for larvae, can increase the detection of infection5,2 and these methods were employed in the present study. However, parasitological methods may fail to detect S. stercoralis, particularly in patients with chronic asymptomatic infection or minimal symptoms.5 It is worth noting that the patients included in the present study had no gastrointestinal symptoms.
It is understood that problems related to the sensitivity of parasitological methods for the detection of S. stercoralis can be solved using molecular methods.16,17,26 The detection of Strongyloides DNA in fecal samples has been the objective of research by several groups,27,28 particularly in cases of immunocompromised patients.29 However, the molecular diagnosis of S. stercoralis infection has been only minimally explored in the context of diabetes. In two recent case reports,15,18S. stercoralis infection in patients with diabetes was confirmed by PCR, suggesting a combination of parasitological and molecular methods for the diagnosis of helminthiasis.30
The present study is the first molecular analysis using two primers for the detection of specific Strongyloides DNA in fecal samples from patients with DM2. The positivity rates were 14.13% and 9.78% by PCR-genus and PCR-species, respectively. Regardless of the target, the positivity of PCR tests was higher than that of the parasitological methods, which has also been confirmed in other studies.28,29 The use of more sensitive methods to detect S. stercoralis infection in endemic areas such as Brazil can minimize the possible complications of severe strongyloidiasis in immunosuppressed patients.4,15
A fundamental step in the application of molecular methods is the choice of targets.26 The present results align with the data presented by Sitta et al.,27 who observed lower quality sequences obtained with the PCR-genus with S. stercoralis sequences present in the database. This can be explained by the amplification of different regions of the ribosomal gene, which is common in other organisms.31 Thus, the possibility of false positives cannot be ruled out, even with the visualization of amplification products with sizes similar to the target fragment, which supports the importance of sequencing.
Furthermore, PCR-species can act as an important tool in the molecular diagnosis of S. stercoralis infection, and the literature has indicated the high sensitivity and specificity of the species-specific primer.16,17,27 A study by Sitta et al.27 evaluating a panel of DNA obtained from fecal samples positive for S. stercoralis, positive for other parasitic infections, and negative, showed superior performance by the PCR-species versus PCR-genus. A species-specific primer was used in conventional PCR and real-time PCR for the detection of S. stercoralis DNA in fecal samples from transplant candidates, and the results showed good diagnostic performance.29 In the present study, all samples with positive amplification for PCR species were confirmed by sequencing to be S. stercoralis.
The potential limitations of this study include the small number of patients with diabetes analyzed and the absence of a control group, which could support the hypothesis of an association between S. stercoralis infection and DM2. However, the results reinforced the high sensitivity of molecular diagnosis in relation to parasitological in the detection of this helminth.
In conclusion, hyperinfection syndrome and dissemination of Strongyloides infection are associated with a high mortality rate, thus emphasizing the need for adequate screening tests to detect helminthiasis when a patient with diabetes has associated diseases that result in immunosuppression. Therefore, molecular methods can be considered an additional tool for the diagnosis of strongyloidiasis, particularly in patients with DM2 who live in areas in which S. stercoralis is endemic.
Authors' contributionsMCM and GBM: Conceptualization, writing (original draft, review, and editing); EAS, LVS, JEO, and BCS: supervision, writing, and visualization; PDM, RCBG, and FMM: writing (draft, review, and editing); FMP and RMR: conceptualization, supervision, writing (review and editing), and visualization.
Financial supportThis work was supported by the researchers themselves.
The authors would like to thank the Secretaria Municipal de Saúde de Jataí, GO, for allowing us to conduct the research, and Dra. Fernanda de Mello Malta for support in the performance of the sequencing of the samples.
ORCID ID: 0000-0001-6156-3649