Subjects exposed to laboratory animals are at a heightened risk of developing respiratory and allergic diseases. These diseases can be prevented by simple measures such as the use of personal protective equipment. We report here the primary findings of the Laboratory Animals and Respiratory Allergies Study regarding the prevalence of allergic diseases among laboratory animal workers, the routine use of preventive measures in laboratories and animal facilities, and the need for prevention programs.
METHODSAnimal handlers and non-animal handlers from 2 Brazilian universities (University of São Paulo and State University of Campinas) answered specific questionnaires to assess work conditions and symptoms. These subjects also underwent spirometry, a bronchial challenge test with mannitol, and skin prick tests for 11 common allergens and 5 occupational allergens (rat, mouse, guinea pig, hamster, and rabbit).
RESULTSFour hundred fifty-five animal handlers (32±10 years old [mean±SD], 209 men) and 387 non-animal handlers (33±11 years old, 121 men) were evaluated. Sensitization to occupational allergens was higher among animal handlers (16%) than non-animal handlers (3%, p<0.01). Accessibility to personal protective equipment was measured at 85% (median, considering 73 workplaces of the animal handler group). Nineteen percent of the animal handlers indicated that they wear a respirator at all times while handling animals or working in the animal room, and only 25% of the animal handlers had received an orientation about animal-induced allergies, asthma, or rhinitis.
CONCLUSIONIn conclusion, our data indicate that preventive programs are necessary. We suggest providing individual advice to workers associated with institutional programs to promote a safer work environment.
Asthma and laboratory animal allergies represent a major occupational illness for thousands of technicians, animal caretakers, physicians, and scientists whose work requires such exposure. Allergy to rats and mice is the most common clinical problem, primarily because these animals are the most widely used in medical research (1). These animals constantly shed proteins via the urine, secretions, and desquamation of skin, and these allergens can be found in the air or deposited on laboratory materials and equipment and make the laboratory a risky environment for the development of allergies (2,3). Work-related sensitization can be associated with the development of skin reactions, rhinitis, conjunctivitis, increased bronchial responsiveness, and asthma (4,5).
Data from cross-sectional studies indicate that 10 to 46% of exposed workers develop laboratory animal allergies (5–10), with symptoms progressing from those of mild rhinitis to more serious symptoms of asthma (5,11). Occupational asthma is a more serious disorder of the lower respiratory system that can result in life-threatening episodes.
Prevention of laboratory animal allergies seems to be crucial for those individuals whose work requires exposure. Although these diseases have the potential to adversely impact both an individual's health and career, a detailed assessment of the routine use of preventive measures in laboratories and animal facilities has not been performed.
To evaluate the need for programs to prevent respiratory and allergic diseases in laboratory animal workers, an epidemiologic study termed LARA (Laboratory Animal and Respiratory Allergies) was designed. The specific aims of this study were to measure the prevalence of asthma, rhinitis, and allergies in workers dealing with laboratory animals; to measure the prevalence of risk factors in this population; to assess the routine use of preventive programs in laboratories and animal facilities; and to create a cohort for follow-up studies on exposed workers and controls.
In this article, we report the primary findings of the LARA study regarding the following 3 objectives: 1) the determination of the prevalence rates of allergic diseases among laboratory animal workers at 2 Brazilian universities; 2) the routine use of preventive measures in laboratories and in animal facilities, including the use of personal protective equipment (PPE) and knowledge about animal-induced allergy, asthma, or rhinitis; and 3) evaluation of the need for preventive programs against allergic and respiratory diseases in laboratory animal workers.
METHODSStudy designData were collected for a cross-sectional evaluation of laboratory and animal facility workers exposed to rats, guinea pigs, mice, rabbits, and hamsters. These workers formed the animal handler group. A group of non-animal handler workers was used as the control group. Data were collected in loco, i.e., in the workplace, from September 16, 2010 to February 10, 2012. All procedures were performed on Wednesdays, Thursdays, and Fridays, thus allowing lung function tests to act as possible indicators of the workweek effect of work exposure. Both groups were evaluated concomitantly. Laboratories and workplaces were randomly selected from the facilities. Two Brazilian universities in different cities were included to obtain more extensive data: the University of São Paulo (USP) and the State University of Campinas (UNICAMP). These public universities are located in the state of São Paulo, Southeast Brazil and respectively consist of 11 and 6 campuses; 1 campus from each university was studied. USP and UNICAMP have 63,927 and 47,674 students, 5,051 and 2,025 professors, and are in the first- and third-ranked positions of the Latin America university system (Quacquarelli Symonds University Rankings), respectively. In the chosen campuses, Ribeirão Preto (RP) and Campinas, there are 10,142 and 36,223 students, 886 and 1,621 professors, and 23 and 37 graduate-level courses, respectively.
PopulationThe animal handler group consisted of subjects engaged in experimental studies, including technicians, students, and researchers. The non-animal handler group consisted of management employees, students, secretaries, computer technicians, car drivers, and others who had no contact with laboratory animals. Cleaning personnel were excluded. Seventy-four (19%) of the non-animal handlers worked in buildings with animal laboratories, although their workplaces were animal-free.
The inclusion criteria were as follows: subjects of both genders, 18 years of age or older, the ability to attend the scheduled visits, and the ability to understand and undergo the procedures. Exclusion criteria included significant non-pulmonary disease, sick leave, and pregnancy.
The entire sample took part in the survey voluntarily. The study was approved by the Ethical Committees of the University Hospital, USP-RP (protocol number 9428/2009) and the School of Medical Sciences, UNICAMP (protocol number 779/2009). Written informed consent was obtained from all subjects.
Recruitment methodsThe laboratories and facilities that work with animals and the employees or students who have direct contact with these animals were first mapped. After this survey, the laboratories to be studied were selected by drawing lots, and each individual was contacted to receive explanations about the study and its risks and benefits.
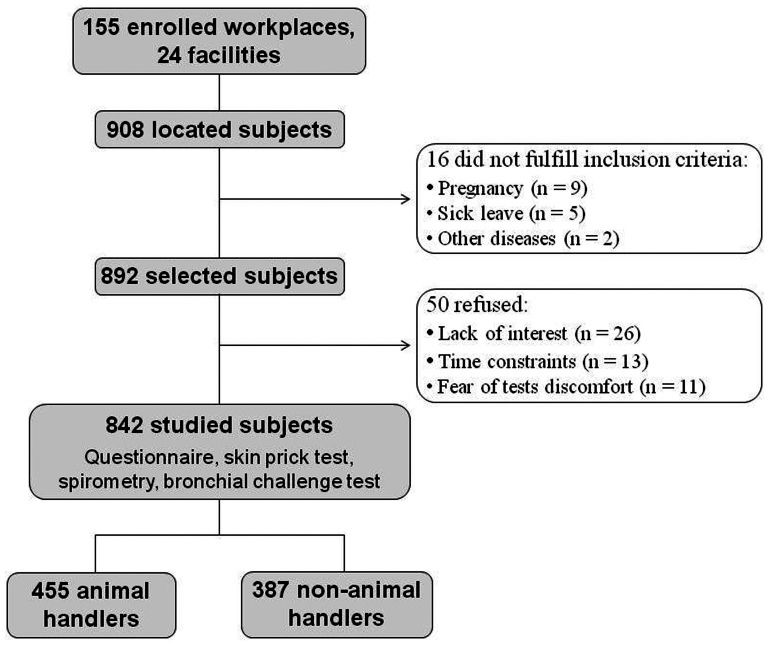
A total of 908 subjects were located at the 155 selected workplaces. Sixteen subjects did not fulfill the inclusion criteria due to pregnancy, sick leave, and other diseases. Thus, the remaining 892 subjects were selected and invited to enroll in the study; of these, 50 (5.6%) refused to participate. The most frequent reasons for non-participation were lack of interest, time constraints, and fear of test discomfort. Thus, the final sample consisted of 842 subjects (94.4% of the selected subjects), including 455 animal handlers and 387 non-animal handlers (Figure 1).
At USP-RP, 247 subjects belonging to the animal handler group were evaluated in 39 workplaces at 8 facilities, and 184 subjects belonging to the non-animal handler group were evaluated in 38 workplaces at 5 facilities. At UNICAMP, 208 subjects belonging to the animal handler group were evaluated in 34 workplaces at 6 facilities, and 203 subjects belonging to the non-animal handler group were evaluated in 44 workplaces at 5 facilities.
QuestionnaireA self-administered questionnaire with 97 questions was used to inquire about respiratory, nasal, ocular and skin symptoms and a personal history of allergic diseases, smoking, and pet owning. The questionnaire items also included job characteristics, such as the duration of working with laboratory animals, job titles, job contents, frequency of contact with animals, species contacted, time spent handling animals, use of protective equipment, and knowledge about animal-induced allergy, asthma, or rhinitis.
Skin prick testSkin prick tests (SPTs) were applied according to the recommendations of the European Academy of Allergology and Clinical Immunology (12). All subjects withheld from taking antihistamine drugs for 15 days prior to SPT.
A wheal diameter of at least 3 mm was considered positive in the absence of a reaction to physiological saline solution and in the presence of a positive reaction to histamine. The allergens included environmental allergens (common allergens, including Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, Felis domesticus, Canis familiaris, Blattella germanica, Periplaneta americana, Alternaria alternata, Cladosporium herbarum, Aspergillus fumigatus, and mixed grass) and extracts from animals (occupational allergens from rats, rabbits, mice, hamsters, and guinea pigs). There were 7 missing values for this variable: 1 subject refused to receive the test because of fear of discomfort, the results of 1 subject were not used due to the absence of a positive reaction to histamine, and 5 subjects used antihistamine drugs prior to the SPT.
SpirometryFor the lung function measurements, participants were asked to avoid smoking and ingesting caffeine for 1 hour prior to the examination, to refrain from using β2-agonists or anticholinergic inhalers for 12 hours prior to the examination, to avoid using oral medications (β2-agonists and theophylline) for at least 24 hours before the test, and to abstain from strenuous exercise for 6 h before the tests (13). Participants reporting an infection of the respiratory system within 6 weeks prior to the procedures were re-scheduled.
Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were measured using a Koko spirometer and software (PDS Instrumentation, Inc., Louisville, Colorado, EUA), which were calibrated daily. Measurements were performed in the sitting position with the subjects wearing a nose clip. At least 3 technically satisfactory maneuvers were attempted for each participant. If it was not possible to obtain at least 3 technically satisfactory maneuvers after 8 attempts, lung function testing was stopped (13). The reference values of Crapo et al. were used to evaluate the results (14). There were no missing values for this variable.
Bronchial challenge test with mannitolDry powdered mannitol (Aridol®) was supplied in kit form (Pharmaxis Ltd., New South Wales, Australia) and contained 1 empty capsule (0 mg), capsules containing 5, 10 and 20 mg, and 15 capsules containing 40 mg (cumulative dose of 635 mg). The dry powder device used for inhalation was the Osmohaler® (Plastiape, Osnago, Italy), a single-capsule device with a low inspiratory resistance. The mannitol challenge required the FEV1 to be measured 60 seconds after each mannitol dose (5, 10, 20, 40, 80, 160, 160, and 160 mg).
The challenge began with the empty capsule, which was loaded into the device and punctured by the investigator. The subjects were asked to inhale from the device from close to functional residual capacity to close to total lung capacity and to hold their breath for 5 seconds. Subjects were encouraged to keep a nose clip on for 10 seconds after inhalation and then exhale through their mouth to minimize deposition in the nasopharynx. In addition to providing the baseline FEV1, the inclusion of the empty capsule demonstrated the sound and use of the device to the subject. Hearing the capsule rotating indicated that sufficient inspiratory flow had been achieved and that it was positioned correctly in the chamber. FEV1 was measured 60 seconds after inhalation of the empty capsule, and the value was obtained and recorded. This value was taken as the baseline FEV1 and was used to calculate the target FEV1 value, which indicated a 15% fall in response to the mannitol challenge. This value was calculated immediately after the administration of the empty capsule.
The first dose of mannitol (5 mg) was administered, and the FEV1 was measured 60 seconds later to obtain the FEV1 value. This procedure was repeated for each dose until a 15% fall in FEV1 was achieved or the cumulative dose of 635 mg had been administered. Based on the findings for healthy non-asthmatics, a 15% decrease in FEV1 to 635 mg or less was regarded as a positive response, as this value was previously shown to indicate bronchial hyperresponsiveness (BHR) (15,16). The cumulative dose of mannitol required to provoke a 15% fall in the FEV1 (PD15) was calculated by interpolation of the log-linear dose-response curve. There were 17 missing values for this variable: 8 subjects refused the test because of the fear of discomfort, 3 tests were interrupted because of cough, and 6 subjects refused the test because of headache and nausea.
DefinitionsAsthmaAn individual was considered an asthmatic subject if he/she exhibited BHR and had experienced symptoms of wheezing, tightness of the chest during the night, or dyspnea during the day or at night in the previous 12 months. Bronchial challenge tests were not necessary to confirm asthma in 14 subjects, as these patients were considered asthmatic based on either a low FEV1 with bronchodilator response or the current use of asthma medication (17). There were 17 missing values for the asthma diagnosis because the bronchial challenge test was not performed.
Probable work-related asthmaAn individual was considered a probable case of work-related asthma if he/she was sensitized to at least 1 occupational allergen (rat, rabbit, mouse, hamster, or guinea pig), had experienced symptoms of wheezing or chest tightness on the job, or was considered asthmatic according to the above definition. There were 17 missing results for the asthma definition, as described above, and 8 additional missing values for this variable: 7 SPT were not performed, and 1 subject did not answer the question regarding symptoms related to his/her job.
RhinitisAn individual was considered to have rhinitis if he/she had experienced symptoms of runny or blocked nose when they did not have a cold in the last 12 months. There were 2 missing values for this variable.
RhinoconjunctivitisAn individual was considered to have rhinoconjunctivitis if he/she had rhinitis (previous item) accompanied by redness and irritation of the eyes. There were 2 missing values for this variable (the same values mentioned above).
Red and irritated skinAn individual was considered to have red and irritated skin if he/she had experienced waxing and waning symptoms of redness and irritation of the skin for at least 12 months. There were no missing values for this variable.
Laboratory animal allergyAn individual was considered to have allergy if he/she was sensitized to at least 1 positive occupational allergen (rat, rabbit, mouse, hamster, and guinea pig) and exhibited any of the following diagnoses: asthma and/or rhinitis and/or BHR. There were 7 missing values for this variable (SPT was not performed).
Quality control proceduresA pilot study with 20 subjects was conducted at both centers to evaluate the comprehensibility of the questions and the time required to answer the questionnaire. Some questions were adapted if the participants had difficulties understanding their meaning.
Adherence to the study protocol was ensured through training workshops with the field staff of the 2 study centers at the beginning and on a regular basis throughout the field phase. All technical and clinical equipment was calibrated regularly. The same individual performed the SPT, spirometry, and bronchial challenge tests at the 2 centers.
Data managementThe questionnaires were coded and typed into a computer by trained individuals according to standardized procedures. The codification was checked by the field supervisor throughout the sampling procedure to detect systematic errors during this phase of the process. Sporadic errors (<1%) within the range expected for this type of study were observed. All of the information typed into the computer was checked. The current database, thus far containing 252 variables of the LARA study, was created in Microsoft-Excel 2003.
Statistical analysisTo determine sample size, we reviewed previous studies that estimated the prevalence of asthma in laboratory animal workers. There are very few published studies, and the reported figures vary up to 30%. Therefore, we selected 20% as a possible prevalence of asthma in laboratory animal workers (18). In previous studies by our group, we described a prevalence of asthma in the general population of approximately 10% (19).
Thus, assuming a level of significance of α = 0.05 and a power (1-β) of 0.90, a sample size of 263 workers per group was deemed sufficient to detect a difference of 10% concerning the prevalence of asthma (20). Our sample was larger than 263 subjects per group because the number of workers in the selected laboratories was larger and because we enrolled everyone to avoid selection bias. In fact, we aimed to enroll at least 90% of the personnel in each selected workplace.
Descriptive statistics were used to determine the demographic data and the data distribution. The Mann-Whitney test and chi-square test were used for comparisons between groups. In all statistical hypothesis tests, the significance level was set at 5%.
RESULTSGeneral dataThis study included 842 subjects, including 455 in the animal handler group and 387 in the non-animal handler group. There was no difference in the proportion of female proportion between institutions and groups (53% of females in USP-RP and 55% in UNICAMP in the animal handler group, and 68% of females in USP-RP and 70% in UNICAMP in the non-animal handler group).
The mean weight of the animal handlers was 71±16 kg (mean±standard deviation), and the mean weight of the non-animal handlers was 69±15 kg. Height also did not differ between the groups (169±9 cm for the animal handlers and 167±9 cm for the non-animal handlers). With respect to the pulmonary function test, 430 (95%) animal handlers exhibited normal spirometry values, and 373 (96%) non-animal handlers exhibited normal values.
Animal handler characteristicsOf the animal handlers (n = 455), 246 were women, the mean age was 32 years, and the current smoking rate was 9% (Table 1). The animal handlers had worked at 14 different facilities, and all had occupational contact with at least 1 of 5 small rodent species (rats, rabbits, mice, hamsters, and guinea pigs). Almost half (44%) of the subjects were sensitized to common allergens, and 16% (n = 74) were sensitized to at least 1 occupational allergen. Of the sensitized subjects (216 cases), 17 (8%) were sensitized only to occupational allergens. Eleven percent (48 subjects) of animal handlers exhibited a laboratory animal allergy, i.e., sensitization plus a symptom/diagnosis. The percentage of women with rhinitis was higher than that of men (66% vs. 49%, p<0.01), as also observed for rhinoconjunctivitis (36% vs. 22%, p<0.01) and red, irritated skin (39% vs. 29%, p = 0.04).
Age and prevalence of outcomes among animal handlers.
| Animal handlers | Gender | Institution | |||
|---|---|---|---|---|---|
| Total | Male | Female | USP-RP | UNICAMP | |
| Outcomes | (n = 455) | (n = 209) | (n = 246) | (n = 247) | (n = 208) |
| Age | 32±10 | 34±11 | 31±9∗ | 32±10 | 32±10 |
| Asthma | 43 (9.6%) | 17 (8.2%) | 26 (10.7%) | 22 (9.0%) | 21 (10.3%) |
| Work-related asthma | 12 (2.8%) | 3 (1.4%) | 9 (3.7%) | 6 (2.4%) | 6 (2.9%) |
| Rhinitis | 263 (58.1%) | 101 (48.8%) | 162 (65.9%)∗∗ | 141 (57.6%) | 122 (58.7%) |
| Rhinoconjunctivitis | 134 (29.6%) | 46 (22.2%) | 88 (35.8%)∗∗ | 68 (27.8%) | 66 (31.7%) |
| Red, irritated skin | 156 (34.3%) | 61 (29.2%) | 95 (38.6%)∗∗ | 91 (36.8%) | 65 (31.3%) |
| Laboratory animal allergy | 48 (10.7%) | 19 (9.1%) | 29 (11.9%) | 30 (12.1%) | 18 (8.7%) |
| Ex-smoker | 40 (8.8%) | 24 (11.5%) | 16 (6.5%) | 19 (7.7%) | 21 (10.1%) |
| Current smoker | 40 (8.8%) | 22 (10.5%) | 18 (7.3%) | 20 (8.1%) | 20 (9.6%) |
| Allergic sensitization | 216 (47.7%) | 100 (47.8%) | 116 (47.5%) | 121 (49.2%) | 95 (45.9%) |
| Sensitization to common allergens | 199 (43.9%) | 92 (44.0%) | 107 (43.9%) | 109 (44.3%) | 90 (43.5%) |
| Sensitization to occupational allergens | 74 (16.3%) | 35 (16.7%) | 39 (16.0%) | 43 (17.5%) | 31 (15.0%) |
| BHR | 58 (12.9%) | 24 (11.6%) | 34 (14.0%) | 32 (13.1%) | 26 (12.7%) |
There were missing values for some variables; see Methods and Definitions. Abbreviations: USP-RP, University of São Paulo at Ribeirão Preto; UNICAMP, State University of Campinas; BHR, bronchial hyperresponsiveness. ∗p<0.01 (Mann-Whitney test); ∗∗p<0.05 (chi-square test).
Of the non-animal handlers (n = 387), 266 were women, the mean age was 33 years, and the current smoking rate was 8% (Table 2). The non-animal handlers worked at 10 different facilities, and their workplaces were free of exposure to laboratory animals. Almost half (47%) of the subjects were sensitized to common allergens, and 3% (12 cases) were sensitized to at least 1 occupational allergen. Similar to the animal handler group, the percentage of women with rhinoconjunctivitis was higher than that of men (32% vs. 22%, p = 0.04), and the percentage of smokers among men was higher than that among women (14% vs. 6%, p<0.01). Allergic sensitization to common allergens was slightly higher at UNICAMP than at USP-RP (52% vs. 41%, p = 0.04).
Age and prevalence of outcomes in non-animal handlers.
| Non-animal handlers | Gender | Institution | |||
|---|---|---|---|---|---|
| Total | Male | Female | USP-RP | UNICAMP | |
| Outcomes | (n = 387) | (n = 121) | (n = 266) | (n = 184) | (n = 203) |
| Age | 33±11 | 33±10 | 33±11 | 35±11 | 31±10∗ |
| Asthma | 36 (9.6%) | 8 (6.6%) | 28 (11.0%) | 13 (7.2%) | 23 (11.8%) |
| Work-related asthma | 1 (0.3%) | 1 (0.8%) | 0 (0%) | 0 (0%) | 1 (0.5%) |
| Rhinitis | 221 (57.1%) | 64 (52.9%) | 157 (59.0%) | 99 (53.8%) | 122 (60.1%) |
| Rhinoconjunctivitis | 112 (28.9%) | 26 (21.5%) | 86 (32.3%)∗∗ | 47 (25.5%) | 65 (32.0%) |
| Red, irritated skin | 124 (32.0%) | 33 (27.3%) | 91 (34.2%) | 62 (33.7%) | 62 (30.5%) |
| Laboratory animal allergy | 8 (2.1%) | 3 (2.5%) | 5 (1.9%) | 3 (1.6%) | 5 (2.5%) |
| Ex-smoker | 28 (7.2%) | 14 (11.6%) | 14 (5.3%)∗∗ | 14 (7.6%) | 14 (6.9%) |
| Current smoker | 32 (8.3%) | 17 (14.0%) | 15 (5.6%)∗∗ | 19 (10.3%) | 13 (6.4%) |
| Allergic sensitization | 179 (46.9%) | 63 (52.1%) | 116 (44.4%) | 74 (40.7%) | 105 (51.7%)∗∗ |
| Sensitization to common allergens | 179 (46.9%) | 63 (52.1%) | 116 (44.4%) | 74 (40.7%) | 105 (51.7%)∗∗ |
| Sensitization to occupational allergens | 12 (3.1%) | 4 (3.3%) | 8 (3.1%) | 7 (3.8%) | 5 (2.5%) |
| BHR | 46 (12.2%) | 12 (9.9%) | 34 (13.3%) | 19 (10.5%) | 27 (13.8%) |
There were missing values for some variables; see Methods and Definitions. Abbreviations: USP-RP, University of São Paulo at Ribeirão Preto; UNICAMP, State University of Campinas; BHR, bronchial hyperresponsiveness. ∗p<0.01 (Mann-Whitney test); ∗∗p<0.05 (chi-square test).
In the animal handler group, there was a higher prevalence of sensitization to occupational allergens (16% vs. 3%, p<0.01). There was no evidence of a difference between animal handlers and non-animal handlers with respect to age (32±10 vs. 33±11 years); the proportion of subjects with asthma (10% in both groups), rhinitis (58% vs. 57%), rhinoconjunctivitis (30% vs. 29%), or red, irritated skin (34% vs. 32%); the proportion of subjects who currently smoke (9% vs. 8%); the proportion sensitized to common allergens (44% vs. 47%); or the proportion with BHR (13% vs. 12%).
Job description of animal handlersThe job characteristics of the animal handlers are described in Table 3. Most selected subjects (80%) worked in animal laboratories rather than in animal rooms; contact with rats (74%) and mice (72%) was the most common type of animal contact. A total of 278 (61%) subjects working with animals were students. The handlers had worked with laboratory animals for approximately 3 years (median), and the weekly exposure of most subjects was more than 8 hours. Approximately two-thirds (63%) of the participants reported having pets, most commonly dogs (86%) and/or cats (20%). Three percent of the participants had owned rabbits, hamsters, or mice in their homes at some point. Forty-three percent reported having worked with laboratory animals in previous jobs, most frequently with rats and mice. The data exhibited small differences in worker populations between the 2 centers; for instance, we detected more full-time workers and more students at USP-RP, where mice and rats were less common, than at UNICAMP, whereas pet ownership was slightly more prevalent at UNICAMP.
Job characteristics of the animal handlers.
| Gender | Institution | ||||
|---|---|---|---|---|---|
| Animal handlers | Male | Female (n = 246) | USP-RP (n = 247) | UNICAMP | |
| (n = 455) | (n = 209) | (n = 246) | (n = 247) | (n = 208) | |
| Type of workplace: | |||||
| Animal room | 91 (20.0%) | 63 (30.1%) | 28 (11.4%)∗ | 42 (17.0%) | 49 (23.6%) |
| Laboratory | 364 (80.0%) | 146 (69.9%) | 218 (88.6%)∗ | 205 (83.0%) | 159 (76.4%) |
| Exposure duration: | |||||
| ≤2 years | 193 (42.6%) | 84 (40.6%) | 109 (44.3%) | 111 (45.1%) | 82 (39.6%) |
| >2 years | 260 (57.4%) | 123 (59.4%) | 137 (55.7%) | 135 (54.9%) | 125 (60.4%) |
| Frequency of animal handling per week: | |||||
| <8 hours/5 days | 87 (19.2%) | 35 (16.7%) | 52 (21.2%) | 35 (14.2%) | 52 (25.0%)∗ |
| ≥8 hours/5 days | 367 (80.8%) | 174 (83.3%) | 193 (78.8%) | 211 (85.8%) | 156 (75.0%)∗ |
| Job: | |||||
| Laboratory technicians | 132 (29.0%) | 83 (39.7%) | 49 (19.9%)∗ | 74 (30.0%) | 58 (27.9%) |
| Office workers | 11 (2.4%) | 6 (2.9%) | 5 (2.0%) | 2 (0.8%) | 9 (4.3%) |
| Students | 278 (61.1%) | 108 (51.7%) | 170 (69.1%)∗ | 165 (66.8%) | 113 (54.3%)∗ |
| Researchers | 34 (7.5%) | 12 (5.7%) | 22 (8.9%) | 6 (2.4%) | 28 (13.5%) |
| Animal species†: | |||||
| Rat | 338 (74.3%) | 162 (77.5%) | 176 (71.5%) | 159 (64.4%) | 179 (86.1%)∗ |
| Mouse | 329 (72.3%) | 153 (73.2%) | 176 (71.5%) | 161 (65.2%) | 168 (80.8%)∗ |
| Guinea pig | 49 (10.8%) | 27 (12.9%) | 22 (8.9%) | 40 (16.2%) | 9 (4.3%) |
| Rabbit | 51 (11.2%) | 26 (12.4%) | 25 (10.2%) | 34 (13.8%) | 17 (8.2%) |
| Hamster | 18 (4.0%) | 11 (5.3%) | 7 (2.8%) | 14 (5.7%) | 4 (1.9%) |
| Pet ownership | 286 (62.9%) | 124 (59.3%) | 162 (65.9%) | 142 (57.5%) | 144 (69.2%)∗ |
There were 1 or 2 missing values for some variables. † Some workers handled more than 1 animal species. Abbreviations: USP-RP, University of São Paulo at Ribeirão Preto; UNICAMP, State University of Campinas. ∗p<0.05 (chi-square test).
The accessibility to PPE among the animal handlers is shown in Table 4. The most accessible type of PPE was gloves (99%), and the least accessible was specific shoes (36%) at both centers. The differences in use between genders and centers may have been due to the types of laboratories studied (e.g., laboratories requiring greater care and involving more complexity). Specific shoes were accessible to 95% of workers in the animal room and to 21% in the laboratories.
Accessibility and reported use of PPE by animal handlers (n = 455).
| Gender | Institution | |||
|---|---|---|---|---|
| Male | Female | USP-RP | UNICAMP | |
| (n = 209) | (n = 246) | (n = 247) | (n = 208) | |
| Accessibility to a respirator | 189 (90.4%) | 211 (85.8%) | 226 (91.5%) | 174 (83.7%)∗ |
| Affirmative answer to the question “Do you wear a respirator all of the time when handling animals or working in the animal room?” | 51 (24.4%) | 35 (14.3%)∗ | 53 (21.5%) | 33 (15.9%) |
| Accessibility to protective eyeglasses | 172 (82.3%) | 194 (79.2%) | 202 (82.1%) | 164 (78.8%) |
| Affirmative answer to the question “Do you wear protective eyeglasses all of the time when handling animals or working in the animal room?” | 22 (10.5%) | 10 (4.1%)∗ | 17 (6.9%) | 15 (7.2%) |
| Accessibility to gloves | 207 (99.0%) | 243 (98.8%) | 247 (100%) | 203 (97.6%) |
| Affirmative answer to the question “Do you wear gloves all the time when handling animals or working in the animal room?” | 153 (73.2%) | 202 (82.1%)∗ | 198 (80.2%) | 157 (75.5%) |
| Accessibility to specific shoes | 98 (46.9%) | 65 (26.4%)∗ | 85 (34.4%) | 78 (37.5%) |
| Affirmative answer to the question “Do you wear specific shoes all of the time when handling animals or working in the animal room?” | 73 (34.9%) | 35 (14.3%)∗ | 46 (18.6%) | 62 (30.1%)∗ |
| Affirmative answer to the question “Have you ever attended a lecture about animal-induced allergy, asthma or rhinitis?” | 14 (6.7%) | 10 (4.1%) | 15 (6.1%) | 9 (4.3%) |
| Affirmative answer to the question “Have you received any verbal orientation about animal induced allergy, asthma or rhinitis?” | 55 (26.3%) | 59 (24.0%) | 65 (26.3%) | 49 (23.6%) |
| Affirmative answer to the question: “Have you read texts, instructions or manuals about animal induced allergy, asthma or rhinitis?” | 57 (27.3%) | 57 (23.2%) | 61 (24.7%) | 53 (25.5%) |
There were 1 or 2 missing values for some variables. Abbreviations: PPE, personal protective equipment; USP-RP, University of São Paulo at Ribeirão Preto; UNICAMP, State University of Campinas. ∗p<0.05 (chi-square test).
PPE was accessible in almost all laboratories evaluated in the study, and 25% of the volunteers stated that they had received an orientation about the importance of using PPE. Nineteen percent of the animal handlers wore a respirator at all times while handling animals or working in the animal room (Table 4). The use of gloves was more common (78%) than the use of other PPE. Overall, the use of PPE by the animal handlers, with the exception of gloves, was below 25% (respirator = 19%, protective eyeglasses = 7%, and specific shoes = 24%). With the exception of gloves, women used less PPE than men, a difference possibly due to the job characteristics of men compared to women. Subjects sensitized to occupational allergens more frequently reported that they wore a respirator than did non-sensitized subjects (Table 5). The use of other PPE did not differ between the groups.
Reported use of PPE by animal handlers (n = 455) with or without sensitization to common and occupational allergens.
| Sensitization to common allergens | Sensitization to occupational allergens | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| (n = 199) | (n = 254) | (n = 74) | (n = 379) | |
| Affirmative answer to the question “Do you wear a respirator all of the time when handling animals or working in the animal room?” | 39 (19.6%) | 46 (18.2%) | 24 (32.4%) | 61 (16.1%)∗ |
| Affirmative answer to the question “Do you wear protective eyeglasses all of the time when handling animals or working in the animal room?” | 15 (7.5%) | 17 (6.7%) | 8 (10.8%) | 24 (6.3%) |
| Affirmative answer to the question “Do you wear gloves all of the time when handling animals or working in the animal room?” | 154 (77.4%) | 200 (78.7%) | 58 (78.4%) | 296 (78.1%) |
| Affirmative answer to the question “Do you wear specific shoes all of the time when handling animals or working in the animal room?” | 46 (23.4%) | 62 (24.4%) | 11 (14.9%) | 97 (25.7%) |
| Affirmative answer to the question “Have you ever attended a lecture about animal-induced allergy, asthma or rhinitis?” | 9 (4.5%) | 15 (5.9%) | 5 (6.8%) | 19 (5.0%) |
| Affirmative answer to the question “Have you received any verbal orientation about animal induced allergy, asthma or rhinitis?” | 59 (29.6%) | 55 (21.7%) | 23 (31.1%) | 91 (24.0%) |
| Affirmative answer to the question “Have you read texts, instructions or manuals about animal induced allergy, asthma or rhinitis?” | 56 (28.1%) | 57 (22.4%) | 22 (29.7%) | 91 (24.0%) |
Common allergens: Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, Felis domesticus, Canis familiaris, Blattella germanica, Periplaneta americana, Alternaria alternata, Cladosporium herbarum, Aspergillus fumigatus, and mixed grass. Occupational allergens: rats, rabbits, mice, hamsters, and guinea pigs. There were 1 or 2 missing values for some variables. Abbreviations: PPE, personal protective equipment. ∗p<0.05 (chi-square test).
In this article, we presented the study design and primary findings of the LARA study, an epidemiological study evaluating the need for programs to prevent respiratory and allergic diseases in 455 laboratory animal workers at 2 Brazilian universities. Our aims were to reveal the prevalence rates of allergic disease among laboratory animal workers, to assess the routine use of preventive measures in laboratories and animal rooms, and to evaluate the need for preventive programs against allergic and respiratory diseases among laboratory animal workers.
Sensitization to occupational allergens was higher among animal handlers (16%) than non-animal handlers (3%), and although access to PPE was observed in 85% (median of all PPEs) of all workplaces, only 19% of the animal handlers stated that they wore a respirator at all times while handling animals or working in the animal room. Moreover, only 25% had received some orientation about animal-induced allergy, asthma, or rhinitis.
The difference in the prevalence of occupational sensitization between handlers and non-handlers (16% vs. 3%) was evident, as was the fact that the prevalence of asthma and rhinitis did not differ between the groups. Nevertheless, in future manuscripts, multiple logistic analyses will be applied to evaluate risk factors, to confirm these findings and to compare specific symptoms such as cough. The importance of this increase in sensitization has been reported in previous studies demonstrating the progression from sensitization to conjunctivitis and rhinitis, as well as asthma (6,18,21,22).
In both groups of subjects, the prevalence of asthma, defined according to BHR and symptoms of wheezing, chest tightness or dyspnea, was 10%, which is similar to the prevalence in the general population (23). However, these data cannot ascertain that the severity of asthma, the frequency of exacerbations, and the asthma prognoses were similar for the 2 groups studied. Moreover, these statistics likely underestimate the incidence of this disease because many workers change jobs when they develop asthma and are not included in cross-sectional surveys.
We defined probable work-related asthma as sensitization to occupational allergens associated with asthma and symptoms of wheezing or chest tightness in the workplace. The precise diagnosis of work-related asthma was not an aim of this study; we sought to identify probable cases of work-related asthma to determine how common this disease may be. On this basis, the prevalence of probable work-related asthma was 2.7% among animal handlers. In a recent study by our group (24), the prevalence of work-related asthma in a cohort of young adults from the general population was 4.2%. Although the definition of work-related asthma differed slightly between these studies, the magnitude of the reported numbers is relevant and considered high by experts around the world (25).
We also identified few differences in the prevalence rates when comparing subgroups of our sample. Women exhibited more symptoms of rhinitis, rhinoconjunctivitis, and red and irritated skin, and the percentage of smokers was lower among women than men (6% vs. 12%, respectively). Gender differences have been discussed in other studies describing similar prevalence findings when comparing men and women (26,27). In the present study, although the smoking frequency was 2-fold higher among men than women, the overall current smoking rates were low.
The difference in the common sensitization prevalence between subjects at USP-RP and UNICAMP was small (41% vs. 52%); this difference does not seem to be relevant and may be the consequence of other variables, such as age, workload, percentage of students, animal species, and pet ownership. Furthermore, occupational sensitization did not differ between USP-RP and UNICAMP. Another important finding regarding sensitization was the fact that all non-animal handler workers with occupational sensitization also exhibited common allergen sensitization; the inverse was also observed in the animal handler group, where occupational sensitization could be isolated from common sensitization. This occurred in 8% of sensitized exposed workers, highlighting the risk of developing allergy to laboratory animals even in workers without previous sensitization to common allergens. Therefore, preventive actions aimed at detecting common sensitization early among workers to relieve them of their duties may not be helpful.
Very few studies have examined the prevention of laboratory animal allergy. In recent years, the occupational incidence of laboratory animal allergy has received increasing attention, and various initiatives have been undertaken to reduce the spread of allergens from animals. Although the focus of control measures may be on individual behavior, some effective practices are related to engineering controls and include housing mice in negatively pressurized ventilated cages, changing the bedding in those cages on pressurized ventilated changing tables, increasing the relative humidity in animal rooms, and covering animal cages with fitted filter-bonnets (8),.
The use of PPE can reduce the allergen exposure of animal handlers as well as protect the animals from infectious agents that may be introduced by skin, clothing, and shoes. Although respirators can provide a high level of protection against laboratory animal allergies (33), only 19% of our animal handlers wore a respirator at all times while handling animals or working in the animal room. The cost of respirators is low compared to engineering controls, although personnel find respirators to be hot and uncomfortable during prolonged use. However, symptomatic animal workers should wear PPE not only in the presence of animals but also when working in facilities where feeding and bedding material are handled and stored. Our data also indicated that sensitized subjects wore a respirator more commonly than did non-sensitized subjects. This difference in safety measure usage may be the consequence of symptoms, and these results indicate that the risk of working with animals is often misjudged, and adequate protection will only be used when the first complaints appear. In the present study, the most available forms of PPE were gloves, and the least available were specific shoes. Specific shoes were not available in all laboratories; furthermore, the use of specific shoes is mandatory only in some animal rooms, where there is a high prevalence of male workers.
The use of protective measures such as gloves, masks, laboratory coats, shoe covers, and respirators was shown to reduce symptoms in 58% of workers with a laboratory animal allergy (34). Moreover, in a longitudinal study published in 1987, Botham et al. (35) demonstrated that introducing measures such as the mandatory use of PPE and the extensive use of educational programs designed to reduce exposure reduced the incidence of laboratory animal allergy from 37% to 12% over a 5-year period. Furthermore, in 2002, Thulin et al. (36) evaluated the effectiveness of safety equipment used to reduce personal exposure in 29 subjects during a period of 6 months and found that only 28% of the subjects used respiratory protection (facial mask) regularly, and 52% used it occasionally. However, by using a ventilated cage-changing wagon, the level of allergen exposure fell from 77 to 17 ng/m3, and these results demonstrated that the introduction of a comprehensive program has the potential to reduce allergen levels.
Surveillance programs should include all personnel exposed or working in close proximity to laboratory animals for 2 or more hours/week and should identify atopic individuals sensitized to domestic or laboratory animals to counsel them on their increased risk of developing laboratory animal allergy (37). Goodno and Stave (38) demonstrated that the incidence of primary laboratory animal allergy was reduced to 0 over the first 5 years of effective preventive practices that included the following: 1) administrative controls, such as control of animal-stock density and the use of wet shaving techniques; 2) environmental controls, such as filter-topped cages, high-efficiency particulate air-filtered room ventilation, increased room air exchanges, and dust-free bedding; 3) PPE, including disposable gloves, bonnets, gowns, and shoe covers, and the mandatory use of respirators (generally dust-mist respirators); and 4) regular housekeeping routines, such as wet mopping and water-hosing.
However, few countries have routinely included these prevention programs as legal requirements. In Germany, Schmid et al. (2009) reported their first experiences with a program based on medical check-ups. These check-ups comprised a questionnaire and a medical examination including pulmonary function tests. Work-related complaints occurred in 33.7% and 37.8% of employees occupationally exposed to mice and rats, respectively, and sensitization rates were 12.7 and 16.3%, respectively (39). These data confirm the necessity of regular medical check-ups for employees in contact with laboratory animal allergens. It is also worth mentioning that the cost of these programs is low compared to the cost of asthma treatment and the associated morbidity.
One of the limitations of the present study was its cross-sectional design, which prevented the establishment of cause-effect relations. However, it will be possible to follow this cohort and re-examine these individuals in the future in an attempt to understand these relationships. Another limitation of a cross-sectional study may involve a “worker's health effect”, i.e., the prevalence of rhinitis, asthma, and sensitization may be underestimated due to abandonment of the activity by subjects with symptoms. In this case, greater numbers of non-susceptible subjects remain in the exposed group and consequently reduce the prevalence rate of allergic disease. To minimize this type of bias, a prospective study needs to be performed.
Among the advantages of our study, we highlight the fact that workplace-based studies generally allow for exposure measures that are not possible in population-based studies, in which participants are derived from a multitude of working environments. In our study, job exposure was assessed according to allergen measurements obtained in laboratories, and this type of ongoing study will allow the quantification of risk estimates. In addition, our study has utilized gold-standard tests for the diagnosis of asthma and allergy. Finally, the sample size obtained will permit statistical analyses of subgroups in future studies.
In conclusion, we found that 16% of animal handlers were sensitized to occupational allergens, whereas only 3% of non-animal handlers were sensitized. The prevalence of asthma was 10% in both groups. We also observed that only 19% of the animal handlers wore a respirator at all times when handling animals or working in the animal room; furthermore, only 25% had received an orientation about animal-induced allergy, asthma, or rhinitis. Therefore, our data indicate that preventive programs for persons with occupational contact with laboratory animals should be mandatory, and we advocate providing individual advice associated with institutional programs for a safer work environment. In addition, employees should be motivated to assume responsibility for their own safety. In most cases, there are options available to reduce exposure and to increase the use of PPE, and additional measures can be implemented by employers. Measures for prevention should include medical check-ups, education, engineering controls, administrative controls, and medical surveillance.
On an institutional basis, our results will help elaborate university policies, educational approaches, laboratory routines, and teaching. Furthermore, our results support the need to pursue research efforts to understand the role of specific preventive measures and to perform cost-effectiveness analyses for the prophylaxis of laboratory animal allergies.
AUTHOR CONTRIBUTIONSFerraz E coordinated data collection, set up database, was responsible for the data analysis and manuscript writing. Arruda LK set up skin prick test and supervised this procedure throughout the study. Bagatin E supervised data collection in the campus of the State University of Campinas. Martinez EZ performed statistical analysis. Cetlin AA was responsible for medical care of volunteers and follow up to confirm diagnosis detected during study. Simoneti CS and Freitas AS performed data collection. Martinez JA and Borges MC helped with protocol and manuscript revising. Vianna EO conceived protocol, managed funding support, tutored data analysis and manuscript writing.
The authors wish to thank all of the participants in this study for their generous contributions as well as the employees, directors, and professors of the laboratories studied. We thank Marcelo Boldrin, Fernanda S. Custódio, and Juliane S. Bedin for assistance with data collection. We also thank Dr. Fernando S. Friestino and Dr. Márcio Z. Cortez for medical assistance and Dr. Satoshi Kitamura for helpful comments on this study. We gratefully acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for funding support.
No potential conflict of interest was reported.