To compare the effects of glimepiride and metformin on vascular reactivity, hemostatic factors and glucose and lipid profiles in patients with type 2 diabetes.
METHODS:A prospective study was performed in 16 uncontrolled patients with diabetes previously treated with dietary intervention. The participants were randomized into metformin or glimepiride therapy groups. After four months, the patients were crossed over with no washout period to the alternative treatment for an additional four-month period on similar dosage schedules. The following variables were assessed before and after four months of each treatment: 1) fasting glycemia, insulin, catecholamines, lipid profiles and HbA1 levels; 2) t-PA and PAI-1 (antigen and activity), platelet aggregation and fibrinogen and plasminogen levels; and 3) the flow indices of the carotid and brachial arteries. In addition, at the end of each period, a 12-hour metabolic profile was obtained after fasting and every 2 hours thereafter.
RESULTS:Both therapies resulted in similar decreases in fasting glucose, triglyceride and norepinephrine levels, and they increased the fibrinolytic factor plasminogen but decreased t-PA activity. Metformin caused lower insulin and pro-insulin levels and higher glucagon levels and increased systolic carotid diameter and blood flow. Neither metformin nor glimepiride affected endothelial-dependent or endothelial-independent vasodilation of the brachial artery.
CONCLUSIONS:Glimepiride and metformin were effective in improving glucose and lipid profiles and norepinephrine levels. Metformin afforded more protection against macrovascular diabetes complications, increased systolic carotid artery diameter and total and systolic blood flow, and decreased insulin levels. As both therapies increased plasminogen levels but reduced t-PA activity, a coagulation process was likely still ongoing.
Cardiovascular disease is the leading cause of mortality in patients with type 2 diabetes mellitus (DM2). The effective control of glycemia can delay but not prevent vascular complications, which are likely related to many other poorly controlled atherogenic factors, such as hyperlipidemia, hypertension, oxidative stress, accelerated aging, hyperinsulinemia, disturbances in coagulation and fibrinolysis (1).
Biguanides and sulfonylureas remain the principal oral therapeutic options for treating patients with DM2 (2). The biguanide metformin has been established as a first-line drug for the management of type 2 diabetes. Its indications are supported by its potency, lack of weight gain, low risk of hypoglycemia and mode of action in countering insulin resistance (3). The drug's anti-atherosclerotic and cardio-protective effects appear to reflect a combination of glucose-independent effects on the vascular endothelium, suppressant effects on glycation, oxidative stress and the formation of adhesion molecules, and anti-inflammatory properties, in addition to stimulating fibrinolysis and favorable effects on lipid profiles (4).
In contrast, sulfonylureas enhance insulin release primarily by closing the ATP-sensitive K+ channels of pancreatic β-cells. The glucose-lowering potency of sulfonylureas is similar to that of metformin. However, by stimulating insulin secretion, sulfonylureas are believed to favor the development of hypoglycemia and weight gain, accelerate beta-cell apoptosis and beta-cell exhaustion and impair endothelial function, thereby increasing the risk for ischemic complications. The blocking of potassium channels in the heart by sulfonylureas has raised concern regarding the drugs' potential adverse effects in cases of ischemic heart episodes (5).
Glimepiride is a long-acting and low potency insulin secretagogue sulfonylurea that has an insulin-sensitizer effect on the muscles and liver (6) and rarely causes hypoglycemia (5). Data from studies in humans are scarce but have suggested that the risks of developing coronary artery disease and mortality do not appear to be increased by glimepiride (7–8). In addition, no deleterious effects of glimepiride on brachial vasodilatation — an acute effect (9) — or ischemic preconditioning — acute (10) and chronic (11) effects — have been observed
Only two studies have investigated the chronic vascular effects of glimepiride measured by forearm arterial blood flow, and the results were similar to those of glibenclamide (12–13) and metformin (12). There have been no reports comparing carotid artery blood flow during glimepiride and metformin therapy, and only a few have measured hemostatic factors (14–19), specifically plasminogen activator inhibitor (PAI-1), fibrinogen and adhesion molecules. In our study, the effects of glimepiride in patients with type 2 diabetes on vascular reactivity, hemostatic factors, and fat and carbohydrate metabolism were compared with those of metformin, the first-line therapy. This comparison is valuable in that these different classes of oral anti-diabetic agents — metformin and glimepiride — target the two main pathophysiological defects of type 2 diabetes, and their safety is fundamental.
METHODSPatientsA prospective study was performed in 16 uncontrolled patients with type 2 diabetes according to the ADA criteria (2); the cohort included ten women and six men with a mean age of 51.8±6.5 years (mean±SD) previously treated with dietary intervention. Subjects who had fasting blood glucose values >7.78 mmol/L and/or glycated hemoglobin exceeding the normal range (4-8.5%) by 1.0% or more after two or more months of a diet therapy program without medications (basal values) were included. The participants were randomly assigned to receive either metformin (M group) or glimepiride (G group). The drug dosage was titrated to achieve fasting glucose levels lower than 7.0 mmol/L using domiciliary capillary glucose measurements. After four months, the patients were crossed over with no washout period to the alternative treatment for an additional four-month period on a similar dosage schedule. The subjects were followed on an outpatient basis every 1-2 weeks throughout the study period for drug and weight-maintaining diet adjustments. The clinical characteristics of the patients are depicted in Table 1. Three of the sixteen subjects smoked, and six had systemic arterial hypertension, which was treated with converting-enzyme inhibitors. Seven of the ten women were postmenopausal, and none had received hormone replacement therapy, while the pre-menopausal women were tested up to the 8th day of the follicular phase of their cycles. At the time of enrollment, a complete medical history, physical examination, and laboratory evaluation, including urinalysis, renal, hepatic, and thyroid function tests and serum lipid and electrolyte levels, were obtained for all of the subjects. An ECG and echocardiogram were performed, and subjects with left ventricular systolic dysfunction, valve abnormalities, arrhythmias and ischemic heart disease were not enrolled. None of the patients exercised on a regular basis. Other exclusion criteria included any severe concomitant illness, nephropathy (serum creatinine >1.6 mg/dL or microalbuminuria), uncontrolled hypertension (BP >190x120 mm Hg), stroke, peripheral vascular disease, marked dyslipidemia (total cholesterol >6.5 mmol/L and triglyceride levels >2.8 mmol/L), coagulopathy, proliferative diabetic retinopathy and use of hypolipidemic and anticoagulant medications. None of the subjects demonstrated clinical evidence of autonomic neuropathy as assessed by blood pressure response to standing, beat-to-beat heart rate variation, the Valsalva maneuver and the handgrip test. The Medical Ethics Committee of Hospital das Clínicas and Heart Institute (INCOR) approved the study protocol, and all of the subjects provided written informed consent.
Anthropometric data.
| Pre-treatment | Metformin Group | Glimepiride Group | ||
|---|---|---|---|---|
| Age (yr) | 51.8±6.5 | |||
| Sex (F:M) | 10:06 | |||
| Dose (mg/day) | 1907±558 | 4±2 | ||
| Weight (kg) | 71.1±13.4 | 68.9±12.5 | 70.5±14.2 | Ns |
| BMI (kg/m2) | 27.6±3.6 | 26.7±3.4 | 27.2±3.7 | Ns |
| W/H | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 | Ns |
| DBP (mm Hg) | 83.3±9.3 | 79.4±7.5 | 81.1±8.5 | Ns |
| SBP (mm Hg) | 141.5±14.0 | 129.2±16.4 | 134.0±14.0 | Ns |
Values are expressed as means±SDs. DBP = diastolic blood pressure; SBP = systolic blood pressure; BMI = body mass index; W/H = waist-to-hip ratio.
The patients were instructed to continue a similar food intake and abstain from the use of tobacco, alcohol, coffee, salty food, and any physical activity for 24 h before test days and discontinue converting-enzyme inhibitors 72 h before the evaluation.
The following procedures were performed before (basal values) and after each four-month treatment period (M and G groups): 1) hormonal and metabolic evaluations: fasting plasma glucose, insulin and catecholamine levels, lipid profiles and HbA1; 2) hemostatic factor determination: tissue plasminogen activator (t-PA) antigen and activity, plasminogen activator inhibitor (PAI-1) antigen and activity, platelet aggregability, fibrinogen and plasminogen levels; and 3) a cardiovascular evaluation: by high-resolution ultrasound of the carotid and brachial arteries.
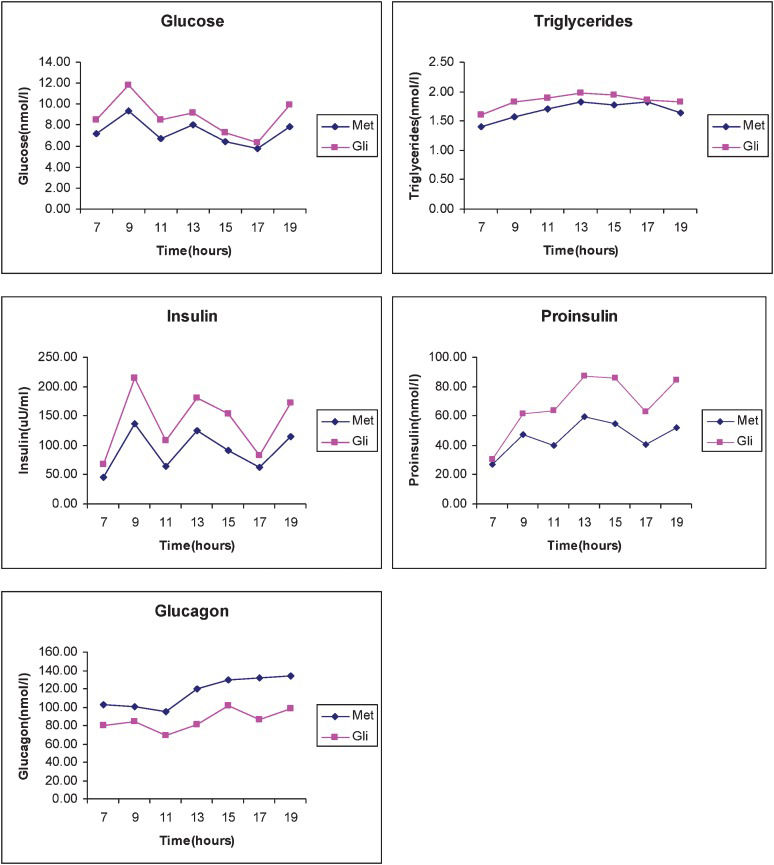
At the end of each treatment period, a 12-h metabolic profile, including measurements of glucose, insulin, glucagon, proinsulin, and triglyceride levels at fasting and every 2 h (from 7 am to 7 pm), was obtained. The meals offered to patients (breakfast, lunch, and dinner) contained 50% of their total calories as carbohydrates, 20% as protein, and 30% as fat, and the meals were provided by the Metabolic Unit of the Hospital das Clínicas.
Cardiovascular evaluationThe study was performed in a laboratory setting at a temperature of 23°C and in low luminosity, with the patients in the supine position. A catheter was inserted into the forearm vein 30 minutes before blood sample collection for catecholamine, insulin, sodium, and potassium determinations and maintained with a saline infusion.
During the study, the heart rate and systolic, diastolic, and mean blood pressure were registered using a non-invasive, automatic oscillometric device (Dinamap 1486, Critikon, Inc., Tampa, FL, USA). The images for flow velocity and diameters of the arteries were first established after a 10-minute equilibration period using a transducer (Apogee-800 Plus, ATL Inc., Bothell, WA, USA). Intima-media thickness (IMT), the compliance and distensibility of the carotid artery, and the total and systolic blood flow indices (TFI and SFI) were obtained. Flow-mediated vasodilation of the brachial artery was measured using a method reported elsewhere (20). A cuff placed on the left forearm was inflated to 200 mm Hg for 5 minutes. Imaging of the artery was performed before cuff inflation (baseline; B) and at 60 seconds after cuff deflation (reactive hyperemia; RH) to obtain measurements of flow velocity and the diameter of the artery. Fifteen minutes after acquisition of the post-occlusion image, the baseline image was reobtained (rebase; RE), after which nitroglycerin was administered sublingually; 3 and 5 minutes later, another image was acquired (N3 and N5, respectively) to establish endothelium-independent vasodilation. The total and systolic flow indices (TFI and SFI) of the brachial artery were also calculated based on these images.
Biochemical and hormonal analysesGlucose was determined by the glucose oxidase method (Labtest, São Paulo, Brazil) (21), and HbA1 (normal values: 4 to 8.5%) was determined by ionic chromatography (Labtest, São Paulo, Brazil) (22). Total cholesterol was measured by employing the cholesterol oxidase/peroxidase method; HDL cholesterol was separated using the phosphotungstic acid/Mg2+ method and measured using the oxidase/peroxidase method, while triglycerides were measured by the lipase/glycerol kinase method (Labtest, Sao Paulo, Brazil) (23). LDL was estimated using the Friedewald equation (LDL cholesterol = total cholesterol minus HDL cholesterol minus 0.2 × triglycerides). The intra-assay and inter-assay coefficients of variation (CVs) for the glucose and lipid determinations were <3% and 0%, respectively. Insulin, proinsulin and glucagon were quantified by a double-antibody radioimmunoassay (Linco Research, St. Louis, MO, USA) (24). Catecholamines were measured by high-performance liquid chromatography (25). The intra-assay and inter-assay CVs for the hormonal analyses were 6.8% and 9.6% for insulin, 4.4% and 6.5% for glucagon, 5.5% and 6.8% for catecholamines, and 5% and 5.3% for proinsulin, respectively.
Hemostatic factors were measured using the same assay. Plasminogen was measured by chromogenic assay (Plasminogen Accucolor TM Sigma Diagnostic, ST Louis, MO, USA), and the intra-assay coefficient of variation was <3.0%. Fibrinogen was determined by the CLAUSS method (26) with Fibriquick Assay (Sigma Diagnostics, ST Louis, USA). The intra-assay CV was 8%. Platelet aggregation was performed using a method described by Born (27). The activities of tissue plasminogen activator (t-PA) and plasminogen activator inhibitor (PAI-1) were determined by quantitative assays (ChromolizeTM t-PA and ChromolizeTM PAI-1, respectively, Biopool, Umea, Sweden); the intra-assay CVs were 3.9% for t-PA and 3.7% for PAI-1. PAI-1 and t-PA antigens were determined by Imulyse and Tint-Elize (Biopool, Umea, Sweden), respectively. The intra-assay CVs were 5% for PAI-1 and 5.5% for t-PA. All of the analyses were performed in duplicate.
Statistical methodsNumerical data are reported as means and standard deviations, and nominal data are reported as proportions. Differences (95% CI) between the treatment groups were initially tested for treatment-time interaction (28) and then compared by an analysis of variance for repeated measurements and a Tukey's post-test or Student's two-tailed test, with p<0.05 considered statistically significant.
RESULTSA significant treatment period interaction effect was demonstrated for triglycerides, VLDL cholesterol, plasminogen and norepinephrine levels; hence, only the values from the first treatment period were analyzed.
Anthropometric, biochemical, hormonal and hemostatic factor measurementsWeight, waist-to-hip ratio, and systolic and diastolic blood pressure did not change after glimepiride or metformin treatment (Table 1). Fasting plasma HbA1 (p = 0.000009) and glucose levels (p = 0.00009) decreased by equal amounts in both treatment groups (Table 2). Fasting insulin levels were greater in the G group (p = 0.009) compared with the M group. Similar decreases in VLDL cholesterol (p = 0.007), triglycerides (p = 0.023) and norepinephrine (p = 0.042) levels were obtained in both groups. There was an increase in plasminogen levels (p = 0.025) after the initial four months of M (118.2±8.2×142.4±32.0) or G (128.4±8.6×130.2±8.1) therapy, which although persistent did not remain significant when the groups switched to the alternative drugs. LDL and HDL cholesterol and epinephrine levels were unchanged. Both therapies decreased t-PA activity (p = 0.024). There was no significant effect of either of the therapies on the other hemostatic factors measured (PAI-1 antigen and activity, fibrinogen levels and platelet aggregation — data not shown).
Glucose, glycated hemoglobin, insulin, total and fractional cholesterol, triglycerides and catecholamine levels.
| Pre-treatment | Metformin Group | Glimepiride Group | p-value | |
|---|---|---|---|---|
| Glucose (mmol/L) | 15.1±5.12 | 7.10±1.34 | 8.3±1.7 | a0.00011 b0.037 |
| HbA1 (%) | 10.8±2.3 | 8.2±1.4 | 7.8±1.3 | a0.009 b0.001 |
| Insulin (pmol/L) | 59.5±22.9 | 55.9±29.4 | 79.6±25.8 | c0.009 |
| Total cholesterol (mmol/L) | 5.41±1.29 | 5.05±0.93 | 5.23±1.09 | Ns |
| HDL cholesterol (mmol/L) | 0.99±0.21 | 1.01±0.18 | 0.95±0.22 | Ns |
| LDL cholesterol (mmol/L) | 3.45±1.16 | 3.32±0.80 | 3.64±0.90 | Ns |
| VLDL cholesterol (mmol/L) | 0.92±0.54 | 0.66±0.31 | 0.70±0.25 | d0.007 |
| Triglyceride (mmol/L) | 2.01±1.19 | 1.38±0.67 | 1.46±0.60 | d0.023 |
| Norepinephrine (nmol/dL) | 1481.0±986.4 | 913.3±497.5 | 958.7±414.7 | d0.042 |
| Epinephrine (nmol/dL) | 104.7±176.8 | 26.1±72 | 16.3±49.6 | Ns |
| t-PA activity (IU/mL) | 1.1±0.5 | 0.7±0.3 | 0.8±0.3 | d0.024 |
Only the areas under the curve during glimepiride and metformin therapy were analyzed (Figure 1). The 12-h integrated areas for glucose (M:87.7±11.03 vs. G:104.61±36.63 mmol/L/h) and triglycerides (M:20.47±9.68 vs. G:22.4±11,03 mmol/L/h) were similar for both therapies.
Treatment with metformin was associated with higher glucagon (M:1361.69±473.25 vs. G:1044.22±326.90 ng/L/h; p = 0.0046) and lower insulin-integrated (M:1076.61±389.02 vs. G:1718.69±837.03 pmol/L/h — p = 0.02) and proinsulin-integrated (M:565.38±279.11 vs. G:834.71±299.96 pmol/L/h — p = 0.0016) areas compared with the G group (Figure 1). The proinsulin-to-insulin molar ratios during the M (0.58±0.32) and G (0.60±0.37) therapies did not differ.
Vascular reactivityThe elastic arterial properties were evaluated.
Carotid artery (Table 3): Both the total (p = 0.004) and systolic (p = 0.002) carotid flow indices increased with metformin therapy compared with baseline values and glimepiride therapy. Moreover, the carotid systolic diameter changed in opposite directions during the therapies; its percentage change (from basal levels) increased with metformin and decreased with glimepiride, and these different behaviors reached significance (p = 0.028). The compliance, distensibility and intima-media thickness of the carotid artery did not change significantly with any therapy (data not shown).
Total (TFI) and systolic (SFI) flow indices and percentage changes in the systolic diameter of the carotid artery.
Brachial artery: There was a similar increase in flow-mediated vasodilatation of the brachial artery after reactive hyperemia (endothelium-dependent) and sublingual nitroglycerin (endothelium-independent) stimuli across the groups. There were no significant effects of medications on systolic diameter or the total and systolic flow indices of the brachial artery (data not shown).
DISCUSSIONThis study compared the actions of two different classes of drugs (the biguanide metformin, an insulin sensitizer, and the sulfonylurea glimepiride, an insulin secretagogue) on carbohydrate and lipid metabolism, hemostatic factors and vascular reactivity. We evaluated the same patients with type 2 diabetes before and after four months of treatment with metformin or glimepiride. The aim of this strategy was to minimize the influence of metabolic control on specific drug effects other than glucose control. The data in the literature remain poor and contradictory regarding the direct actions of these drugs on the cardiovascular risk-related factors analyzed in the present study (15–19).
Carbohydrate and lipid metabolismBoth treatment groups achieved similar and significant mean decreases from baseline in fasting plasma glucose and HbA1 levels. The lower insulin and proinsulin levels (at fasting and during the 12-h metabolic profile) observed during metformin therapy compared with glimepiride were in agreement with reports of metformin's sparing effects on beta cell function, thus lowering the basal and postprandial insulin requirements for the same metabolic control (3,17). These differences in insulin secretion could not be accounted for by changes in body weight, which were unaffected in both groups. Although some authors have found no increases in insulin levels (18) and others have reported that glimepiride's insulin trophic effect might diminish in the presence of normoglycemia (34), the insulin levels in this study were found to be higher during therapy with glimepiride than metformin.
Despite these findings, the proinsulin-to-insulin ratio areas under the curve during the 12-h metabolic profile were similar for both therapies, suggesting that glimepiride did not worsen the previous secretor dysfunction of beta cells, as reported with other sulfonylureas (35).
The overall effects on plasma lipids were small, with similar lowering of fasting VLDL cholesterol and triglyceride levels after the initial four months of both therapies. TG levels during the 12-h metabolic profile were also similar between the two drugs. LDL and HDL cholesterol levels were unaffected by treatment. Metformin. and glimepiride-associated improvements in lipid metabolism were expected, but the reported changes have been small (3,16,30). The near normal triglyceride and cholesterol levels of our patients prior to both therapies were likely factors that influenced these modest results.
Despite causing lower insulin and higher glucagon secretion, metformin kept glucose and lipid profiles at similar levels compared with glimepiride. The blood glucose-lowering actions of metformin result primarily from an amelioration of insulin resistance, increase in peripheral glucose disposal, decrease in fatty acid oxidation and activation of the enzyme adenosine monophosphate (AMP) kinase to increase glucose transporter 4 (GLUT4) translocation to plasma membrane cells in the muscles and fat and reduce gluconeogenesis in the liver (3,36). The higher glucagon levels are unlikely to have interfered with metformin's action, as glucagon appears to have little effect on the presence of insulin, suggesting that its diabetogenic action occurs only under conditions of high insulin deficiency (37). In addition, glucagon's effects on hepatic glucose production could have been strongly counteracted by metformin.
Hemostatic factorsGlimepiride and metformin had similar effects on hemostatic factors; both increased plasminogen levels (significant after four months on each therapy) and decreased t-PA activity. These findings observed in the G group have not been reported previously.
The improvements in fibrinolysis after metformin and glimepiride therapy suggested by the increases in plasminogen levels occurred along with an unexpected decrease in another marker of fibrinolysis — t-PA activity. In contrast to our results, an increase in t-PA activity during metformin therapy has been described (38). However, as active t-PA declines as a function of increasing concentrations of PAI-1 and considering that PAI-1 antigen and activity did not change in our experiment, we can speculate that the formed complexes of PAI-1 and t-PA cleared in an accelerated fashion by the liver (39) contributed to the decrease in t-PA activity and that an impairment of fibrinolysis continued to occur. These data are likely implicated in the high frequency of cardiovascular disease in type 2 diabetes. No additional effects of any of the therapies were observed on the other hemostatic factors analyzed (fibrinogen levels and platelet aggregation — data not shown).
Vascular reactivityIncreased carotid artery systolic diameter and blood flow were demonstrated only with metformin therapy, whereas an opposite trend in carotid diameter was observed with glimepiride use. Similar trends were observed even after patients treated for hypertension were excluded. No changes were observed for the other properties of the carotid (compliance, distensibility, and intima-media thickness) or brachial arteries (flow and diameter measurements after stimulus with reactive hyperemia and nitroglycerin).
Metformin's effects on carotid artery flow, independent of glucose decay or changes in systolic and diastolic blood pressure, likely afford more protection against cerebral diabetes complications. Its countering of insulin resistance action is likely implicated. Furthermore, we observed a negative correlation between fasting serum insulin levels and carotid compliance in the M group (r = -0.5; p = 0.04). Several observational analyses have suggested cardio-protective benefits with metformin use in patients with cardiovascular disease (3–4),. Patients on metformin exhibited reduced nitroxidant metabolites and increased nitric oxide levels (30). As the carotid artery is more elastic and more proximal to the heart than the brachial artery, it might be more amenable to these improvements.
As both therapies improved glucose control in very similar manners, their beneficial effects on lipid profiles, hemostatic factors and norepinephrine levels can likely be ascribed to improvement in the metabolic milieu and not to a specific drug effect. Our study is the first to demonstrate decreases in norepinephrine levels after the first four months of glimepiride therapy and similar trends for epinephrine levels. The same results were obtained for metformin.
Although metformin has been confirmed as the first-line option for treating diabetes, troublesome gastrointestinal intolerance occasionally precludes its use (3). Thus, sulfonylureas remain important adjuvants for patients with intolerance to metformin or limited insulin secretion (5). When compared with metformin, glimepiride achieved similar efficacy in controlling weight and improving metabolic and hemostatic factors. Although it led to greater insulin secretion, glimepiride did not worsen beta cell function, as measured by the proinsulin-to-insulin ratio or vascular reactivity. Long-term studies are needed to ascertain whether glimepiride can reduce beta cell exhaustion or apoptosis.
Our study has some limitations. The four-month treatment duration could have not been sufficient to demonstrate all of the effects of these medications. Additionally, as a crossover study with no washout period, a treatment period interaction effect was demonstrated for some variables (triglyceride, VLDL cholesterol, plasminogen and norepinephrine levels). To minimize this problem, only the values from the first treatment period were analyzed.
In patients with type 2 diabetes inadequately controlled by dietary therapy, M and G resulted in similar overall improvements in glycemic control, lipid profiles, norepinephrine levels, and levels of the fibrinolytic factor plasminogen. All of these beneficial effects were likely due to improvement of the metabolic environment and were not drug-specific. However, as both therapies reduced t-PA activity, the coagulation process continued, which can worsen cardiovascular disease. Only metformin countered insulin resistance and induced an increase in carotid artery diameter and blood flow indices. Neither drug affected small brachial artery vasodilation.
AUTHOR CONTRIBUTIONSMachado HA and Cunha MR participated in designing the studies, conducting the clinical and experimental procedure and writing the paper. Vieira M provided the cardiovascular evaluation. Correia MRS performed the biochemical analysis. Fukui RT performed the hormonal analysis. Santos RF, Wajchenberg BL and Rocha DM collaborated on the clinical study. Lage SG collaborated on the clinical study and the cardiovascular evaluation. Silva MER collaborated on the clinical study and wrote, edited and finalized the manuscript for publication.
We are grateful to Greci de Paula Souza for assistance with procedures. This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP.
No potential conflict of interest was reported.