Pandemics impose large demands on the health care system. The supply of appropriate chemotherapeutic agents, namely oseltamivir solution, presented a serious challenge in the recent influenza pandemic. This study reports on the rational series of pharmacotechnical steps that were followed to appropriately handle bulk oseltamivir powder to meet the increased demand.
METHODS:During a six-week period in August and September of 2009, a task force was created in the Central Pharmacy of Hospital das Clínicas to convert imported oseltamivir phosphate into ready-to-use solution for utilization by physicians and public health authorities. The protocol included dissolution, physico-chemical tests and the bottling of a liquid microdose formulation for emergency room and outpatient dispensing with adequate quality control during all phases.
RESULTS:The successful production routine was based on a specially designed flowchart according to which a batch of 33210 g of oseltamivir powder was converted into 32175 solution units during the aforementioned period with a net loss of only 2.6%. The end products were bottles containing 50 ml of 15 mg/mL oseltamivir solution. The measured concentration was stable and accurate (97.5% - 102.0% of the nominal value). The drug was prescribed as both a prophylactic and therapeutic agent.
DISCUSSION:Hospital pharmacies are conventionally engaged in the manipulation of medical prescriptions and specialty drugs. They are generally responsible for only small-scale equipment used for manufacturing and quality-control procedures. The compounding of oseltamivir was a unique effort dictated by exceptional circumstances.
CONCLUSION:The shortage of oseltamivir solution for clinical use was solved by emergency operationalization of a semi-industrial process in which bulk powder was converted into practical vials for prompt delivery.
During the last 400 years, there has been an influenza epidemic approximately every three years and a pandemic about three times every century. In recent times, the Spanish Influenza of 1918-1919 is best remembered, which resulted in a lethality of about 0.5% and up to thirty million deaths worldwide.1 This unfavorable behavior is explained by frequent mutation of the viral genome and possible reassortment with genetic material from related viruses in animal hosts, which may permit evasion of the host immune response.
The current swine influenza A is a variant of the H1N1 influenza strain that was responsible for the Spanish Influenza. Fortunately, the current pandemic strain responds to oseltamivir, a neuraminidase inhibitor that reduces symptoms and accelerates recovery.2,3
According to directives of the Brazilian Ministry of Health and in agreement with the World Health Organization, institutions in various parts of Brazil were charged with the local production of oseltamivir during the 2009 pandemic. Our institution has published a number of reports on the management of this outbreak.4-6 The Central Pharmacy of Hospital das Clinicas, São Paulo (CPHC), was assigned the responsibility of preparing the oseltamivir solution.7 The CPHC met this unusual demand without overlooking pharmacotechnical safety and accuracy or the requirements of good manufacturing practices (GMP).8
These concerns are not exceptional within the pharmaceutical industry because they are addressed on a daily basis by large and specially trained teams. The challenge is greater within hospital pharmacies, which have less abundant labor and resources as well as less experience in handling such unconventional tasks. If one adds the urgency of a serious epidemic, then the equation becomes even more complex. Nevertheless, the mission of the CPHC was to prepare multiple batches of oseltamivir solution for oral administration to affected patients at short notice.
The aims of this study are to present the processes and controls adopted by the CPHC that led to the successful achievement of the set targets.
METHODSPlanning and executionWithin the period of August 6-September 16, 2009, an emergency task force was defined with the goal of preparing oseltamivir phosphate vials for clinical use. The main responsibility was assumed by the pharmacists of the Pharmacotechnical Unit of the Division of Pharmacy, Hospital das Clinicas. The sector of non-sterile oral fluids was mobilized, and selected professionals were assigned to production, quality control and supervision responsibilities.
The compounding process was based upon the process described in the product literature provided for oseltamivir by Roche, Sao Paulo, Brazil, 2 which included basic information about the salt and its handling. A protocol that included raw material testing, characterization, dissolution, bottling and packaging was drafted as a flowchart. Progress along the flowchart was monitored by the appropriate physico-chemical tests, and full accountability was provided by means of careful registration of the final products, yields and losses, in agreement with GMP.
Production targetThe production end point was bottles containing 50 ml of 15 mg/mL oral Oseltamivir solution (Table 1). This concentration is the German standard, as described in the German National Formulary NRF 31.2.3 A minor discrepancy with standard manufacturer recommendations can be noticed (12 mg/mL instead of 15 mg/mL); however, this dose was considered the most convenient in the pandemic context. (Table 2).2,3
Composition of the Oseltamivir bottles defined by the Central Pharmacy of Hospital das Clínicas.
| Ingredient | Amount |
|---|---|
| Oseltamivir phosphate | 985 mg ∗ |
| Sodium benzoate ∗∗ | 50 mg |
| Distilled water ∗∗∗∗ | 50 ml ∗∗∗ |
(∗) Equivalent to 750 mg of oseltamivir; Final concentration 15 mg/mL; (∗∗) Preservative agent; (∗∗∗) Actual volume 51 ml in order to comply with legislation; (∗∗∗∗): Volume sufficient for 50 ml;
International guidelines for quality control were strictly adopted.9 These guidelines included the analysis of raw materials, packaging and finished products by the quality control pharmacist. The identification and inspection of raw materials was routinely conducted, including initial high performance liquid chromatography (HPLC) testing.
Storage conditions and handling precautions followed manufacturer instructions. Safe packaging materials were employed (amber-colored prescription bottles), and finished products were examined for cracked or broken units and incorrect labeling. Batch processing records included the name of the product, lot number, time of start and completion of the compounding process, total yield, accidental losses and the name of the professional in charge. All deviations from specified procedures were registered and reviewed.
Finished lots were quarantined until analytical and general quality control procedures were completed and filed, and samples were retained. The finished lots were only released after certification by the responsible pharmacist.
Weekly post-production controls of oseltamivir molecular content were conducted by HPLC analysis of production lot samples according to the following schedule: 0 (immediately after compounding), 7, 14, 21 and 28 days after compounding.
HPLC protocolThe Varian Auto-Sample ProStar 410 HPLC system (Varian Inc., Palo Alto, CA, USA), equipped with a ProStar 325 UV-Vis photodiode array detector, ProStar 500 column valve module, and ProStar 230 solvent delivery module was used for method development and validation. A Microsorb-MV C8/5-µm (4.6 × 250 mm) column with a mobile phase of phosphate buffer (pH 6; 0.05 M), methanol and acetonitrile (620:245:135, v/v) was used at a flow rate of 1.0 mL/min. Sotalol hydrochloride (Bristol-Myers-Squibb, São Paulo, Brazil) was selected as an internal standard, and an oseltamivir phosphate standard sample (Roche, São Paulo, Brazil) was selected as the external standard. Galaxie software (Varian, Palo Alto, CA, USA) was employed for data acquisition. This procedure was adapted from previously published protocols.3,10
RESULTSManufacturing and quality-control processesThe principal pharmacotechnical procedures are described in Table 3.
Manufacturing flowchart.
| Material | Features and purposes | Routines |
|---|---|---|
| Solid ingredients | Raw materials | Weights, physico-chemical properties, date and time of arrival and processing, expiry date |
| Oseltamivir phosphate | Active substance | Net drug content, solubility, pH, fusion point |
| Distilled water | Dissolving medium | pH, total oxidants, electrical conductivity, other physico-chemical properties, lot and time of preparation |
| Dissolution and homogenization | Oseltamivir phosphate and sodium benzoate (preservative) | Mixing in steel reactor, filtration, pH, particulate matter and visual inspection |
| Final product | End point | Lot, packaging, labeling and storage, shelf life |
| Residues and rejected materials | Useless and hazardous byproducts | Registration of weights, volumes and materials, discarding according to GMP and environmental legislation |
| Documentation | Quality Control and GMP | Initial and final time of each operation, yield, losses, packaging, labeling and storage, protocol nonconformities and technical problems |
Abbreviations: GMP: Good manufacturing practices;
A total batch of 33,230 g of oseltamivir phosphate was received. This amount of powder was sufficient to produce 9 lots of the solution for a total of 32,175 units of oseltamivir solution with a net loss of 2.6%.
During 2009, oseltamivir represented about one-quarter of the total output of the non-sterile oral fluid unit of the Division of Pharmacy. The actual percentages according to released drug bottles or packages were as follows: potassium chloride syrup, 25.8%; oseltamivir phosphate, 25.1%; codeine drops, 9.4%; aluminum hydroxide, 9.2%; liquid Vaseline, 7%; sodium bicarbonate, 6.9%; 50% oral glucose solution, 4.5%; and miscellaneous drugs, 12.1%.
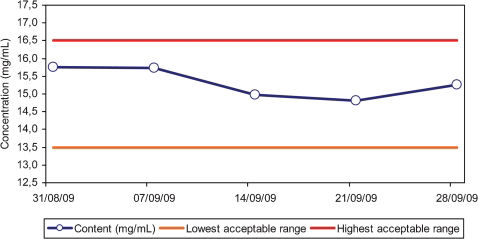
Several techniques adopted in the production flowchart can be followed in Figures 1-3. The shelf life of the drug was established as 21 days, although evaluations indicated excellent stability for longer periods (Figure 4).
Molecular contentThe actual concentration of oseltamivir corresponded to 97.5%-102.0% of the nominal value, which is within the acceptable range for pharmacological and legal purposes.3,11,12
DISCUSSIONThe handling of an oral pharmaceutical solution is not only a matter of solubility, dosage and excipients. Multiple other variables, such as characterization of the dissolved drug, stability, dose-uniformity, and palatability or the absence of chemical irritants, are also important.13
Ingredients must be suitable and compatible, and appropriate physical and physico-chemical control methods must be applied to ensure technical success and to comply with quality control routines.
GMP regulations have been accepted worldwide and must be followed. They encompass the design, production, storage and disposal of the agent, related chemicals, solvents and other materials. An important feature of such practices is full accountability of all manipulation steps.9,11,12
Raw materials, intermediates, and final products should be weighed and measured or calibrated to ensure accurate results within appropriate ranges. Complete documentation at each step of manufacturing is required. Actual yields and percentages of theoretical yields must be determined at the conclusion of each phase of processing, packaging, and storage of the drug solution, and the data must be saved in specific files. All calculations require independent confirmation, with the report of a single professional being insufficient.9,11,12
Oseltamivir phosphate is a precursor of the active metabolite oseltamivir carboxylate, which is a selective inhibitor of influenza A and B neuraminidase enzymes. Neuraminidase participates in viral entry into the cell and in the subsequent release of virus particles and dissemination of the infection. Extensive worldwide experience confirms its value both in the prophylaxis and treatment of endemic and epidemic influenza outbreaks.2
Oseltamivir is licensed for use in all groups with the exception of pediatric patients. Dosage flexibility makes it ideal for epidemics, and although compounding of capsules was not overlooked by the Brazilian Ministry of Health, much emphasis was placed on the rapid release of the solution formulation.7,10
In the report by Albert and Bockshorn, refrigerated samples of extemporaneously prepared oseltamivir solution were stable for 84 days, as validated by HPLC. However, due to the possibility of toxic metabolites, this limit was lowered to 46 days. In contrast to sterile water, the pH gradually increased and shelf life diminished to 14 days with the formation of a precipitate when potable water was selected.3
Winiarski et al. reported worse results when converting oseltamivir capsules into oral solutions. Although high-quality pharmacotechnical procedures were adopted, the stability under refrigeration was not more than 35 days, and it decreased to as little as 5 days at room temperature.10
In the current study, excellent stability was demonstrated for at least 21 days at room temperature, with no detection of metabolites by HPLC, change in pH or formation of precipitates.
The results demonstrate that the product was successfully processed and manufactured, with careful documentation of each technical step, and that there were negligible errors or losses. The hurdles of a comparatively small team and limited resources were circumvented without compromising the quality or reliability at CPHC, which is not a standard industrial facility. As mentioned above, its institutional role is the compounding of commercially unavailable drugs along with special formulations for patient use within the 2,000–bed hospital complex.
The 2009 outbreak now looks mild and is partially forgotten; however, it was a major issue in the initial months of the pandemic. “Swine flu” was first reported in Mexico on April 26, 2009. Within less than 50 days, on June 15, it had assumed pandemic proportions with 35,928 cases and 163 deaths in 76 countries.14 Repeated announcements by the World Health Organization to all member states warned about a problem of large proportions.
It was predicted that if the virus achieved equivalent virulence to that of the 1918-19 pandemic strain, 62 million people could die. Emergency alerts included travel advisories, stockpiling of antiviral drugs, vaccine development, and planning for business continuity and maintenance of essential services.15
Various countries mobilized their disaster management authorities,15 eventually including the Armed Forces,16 to control the outbreak of the swine flu. The European Society of Intensive Care Medicine established a task force and issued a special supplement of its journal, defining recommendations and standard operating procedures for dealing with the pandemic, which had the potential to progress to a massive disaster.17,18
Emergency departments in New York hospitals were overwhelmed in April and May of 2009, and authorities braced for a public health crisis.19 All of these facts notwithstanding, the priorities were epidemiological (information exchange and biosurveillance efforts), clinical (screening and therapy) and logistical (manpower, infrastructure and drug stockpiling).14-19 No report regarding pharmacotechnical bottlenecks and how to handle them has been found in the literature.
The principal contribution of this study was not the creation of innovative manufacturing processes or quality control criteria, as these were mostly adapted from published guidelines and pharmacopeias as well as from existing CPHC routines.2,3,9-12 Instead, the advance was achieved by the prompt reaction to the emergency challenge and the fulfillment of a labor-intensive and exacting mission within a nontraditional framework of pressing time schedules, heavy workloads and limited resources.
Nevertheless, weaknesses in this production process should be noted. Production glitches, accidents including the development of impurities and precipitates, and drug losses adversely influenced the product yield and shelf life. As shown, catastrophes were avoided and end points were quite successfully reached, demonstrating that indispensable pharmacotechnical standards must be adhered to at all times.
This unique experience could serve as a model for emergency episodes in the future in case similar difficulties emerge. It should also stimulate the improvement of the existing infrastructure and contingency plans so that daunting or last-minute tasks are minimized. Preparedness is a requirement for all countries. In recent times, military strategic thinking has trickled down to civilian life, including the health professions.
Every infectious outbreak exhibits different features and circumstances, and people should be careful not to stockpile dated ammunition or to fight yesterday's war. One of the most important components of the preparedness of any society is “institutional memory”; it is important that lessons and abilities taught by past events be remembered. Such intellectual and technical patrimony should not be simply documented and filed; instead, it should be critically examined and periodically updated so that it is ready to be retrieved at any moment.
CONCLUSIONA slow step-wise approach would be the most sensible choice for the adoption of a new process such as the preparation and use of oseltamivir solution; however, the health care system needed the drug immediately. An urgent solution was thus adopted, with simultaneous planning, testing and execution of all techniques, following initial studies for the development of pharmacotechnical methods and procedures. Although this approach is not recommended as a template for ordinary circumstances, important lessons were learned about reactivity and rational crisis management within the hospital pharmacy.