Kidney transplantation corrects endocrine imbalances. Nevertheless, these early favorable events are not always followed by rapid normalization of parathyroid hormone secretion. A possible deleterious effect of parathyroidectomy on kidney transplant function has been reported. This study aimed to compare acute and long-term renal changes after total parathyroidectomy with those occurring after general surgery.
MATERIALS AND METHODS:This was a retrospective case-controlled study. Nineteen patients with persistent hyperparathyroidism underwent parathyroidectomy due to hypercalcemia. The control group included 19 patients undergoing various general and urological operations.
RESULTS:In the parathyroidectomy group, a significant increase in serum creatinine from 1.58 to 2.29 mg/dl (P < 0.05) was noted within the first 5 days after parathyroidectomy. In the control group, a statistically insignificant increase in serum creatinine from 1.49 to 1.65 mg/dl occurred over the same time period. The long-term mean serum creatinine level was not statistically different from baseline either in the parathyroidectomy group (final follow-up creatinine = 1.91 mg/dL) or in the non-parathyroidectomy group (final follow-up creatinine = 1.72 mg/dL).
CONCLUSION:Although renal function deteriorates in the acute period following parathyroidectomy, long-term stabilization occurs, with renal function similar to both preoperative function and to a control group of kidney-transplanted patients who underwent other general surgical operations by the final follow up.
Secondary hyperparathyroidism (HPT) is an almost universal complication in patients with chronic renal failure. Successful kidney transplantation corrects endocrine and metabolic imbalances and the main abnormalities responsible for secondary HPT in the first months following surgery.1 Nevertheless, these early favorable events are not always followed by a rapid normalization of parathyroid hormone (PTH) secretion. Elevated PTH levels are observed in up to 25% of patients one year after transplantation, despite adequate renal function.2 A subgroup of these patients will ultimately require parathyroidectomy.
A possible deleterious effect of parathyroidectomy on kidney transplant function was reported.3 Evidence of an acute effect of parathyroidectomy without significant compromise in the long run was reported.4 In one study, the only parameter observed to correlate with an acute decrease in renal function was an acute reduction in PTH.5
In one study of successfully kidney-transplanted individuals, renal changes after total parathyroidectomy with immediate autograft, which were estimated by variation in creatinine level, differed in comparison to changes in kidney transplant patients undergoing other head and neck procedures.6 In that study, the control group included only a small number of patients, and observation was restricted to the first days after the operation. These aspects limit the conclusions that can be drawn regarding the long-term safety of total parathyroidectomy in the management of persistent HPT after kidney transplantation. Although kidney dysfunction has also been described in patients undergoing subtotal parathyroidectomy,7 one study suggested the superiority of this technique over total parathyroidectomy with autotransplantation in the management of persistent HPT after kidney transplantation.8 For some renal transplant patients, it was recommended that total parathyroidectomy should be avoided to avoid compromising long-term renal function.8
The present study aimed to compare acute and long-term renal changes in patients undergoing either total parathyroidectomy and immediate autotransplantation or comparable general surgical operations in a larger subset of kidney transplant patients.
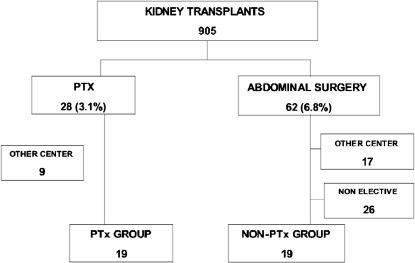
METHODSThe study was a retrospective case-controlled study of renal allograft recipients at a single institution. This study was part of a project approved by the Institutional Ethical Committee on research. Records from all patients who received a kidney transplant at the University of São Paulo, Hospital das Clinicas, between January 2000 and December 2006 were reviewed. A total of 905 patients were analyzed which may be compared to the 715 patients who received kidney transplants in the five year period 2002-2007 covered by the official registry of the Institution.9 Kidney transplant patients who underwent a total parathyroidectomy with autotransplantation for persistent HPT or for HPT developed after transplantation were designated the case group. The control (non-parathyroidectomy) group consisted of renal transplant cases submitted to elective abdominal operations. Figure 1 shows patient selection. The following subjects were excluded from the final analysis: patients with infected abdominal surgeries and patients who received their parathyroid or abdominal surgeries at other centers.
The demographic data, cause of renal failure, type of renal pre-transplant replacement therapy, type of donor, immunosuppressive therapy, dialysis duration before transplantation, time from transplantation to surgery, serum creatinine levels and estimated creatinine clearance were analyzed. Creatinine clearance was estimated using the Cockroft-Gault equation. Creatinine levels were compared at baseline (i.e., before the operation), in the early postoperative period (i.e., the highest value obtained during the first week after the operation) and in the long-term follow up. All patients were followed until the first occurrence of renal transplant failure (defined as the need for renal replacement therapy or preemptive renal retransplantation), death or 1 January of 2007 (end of study).
Statistical analyses for continuous variables are presented as mean ± standard deviation in cases in which the distribution is parametric. Student's t test was employed to verify possible differences between groups. Contingency tables were created for qualitative variables and were tested by Fisher's Exact Test or the chi-square test, as appropriate.
RESULTSBetween January 2000 and December 2006, 28 of 905 renal transplant patients at our transplant outpatient clinic underwent parathyroidectomy because of persisting hyperparathyroidism, resulting in a period prevalence of 3.1%. Nine patients were excluded from the evaluation because they underwent parathyroidectomy at other centers. During the same period, 62 patients underwent abdominal surgery. Of those, 26 had non-elective operations, and procedures took place in other centers; these were excluded from the present analysis.
The 19 patients with persistent HPT underwent parathyroidectomy due to hypercalcemia. The 19 patients in the control group received general and urological operations as follows: herniorrhaphy in 9, radical prostatectomy in 4, transurethral prostatectomy in 2, 1 cholecystectomy, 1 laparoscopic nephrectomy, 1 open nephrectomy plus cholecystectomy and 1 open herniorrhaphy plus cholecystectomy.
Renal replacement therapy before transplantation consisted of hemodialysis in 95% of the parathyroidectomy group and 89% of the control group. None of the cases in the study group underwent peritoneal dialysis before transplantation as compared with 5% of the control group. The remaining patients in both groups had pre-emptive renal transplantation, with no significant differences in mean time on dialysis or mean time of surgery after renal transplantation. The mean time on dialysis before renal transplantation was 86.1 (range 0-204) months in the parathyroidectomy group and 57.2 (range 0-120) months in the control group (p = 0.047). The mean time for surgery after renal transplantation was 34 months (range 7–69 months) for the parathyroidectomy group and 26.9 months (range 3-76) for the control group (p = 0.23).
The cause of chronic renal failure was glomerulonephritis or vasculitis in 47% of the patients undergoing parathyroidectomy and in 37% of the controls. Hypertensive disease induced renal failure in 26% of the study cases and in 21% of the controls. Cystic, hereditary or congenital diseases were found in 11% of the cases and in 21% of the controls. Diabetes was not observed in any of the study cases but was present in 5% of the controls. The etiology of renal failure was unknown or missing in 11% of the patients who underwent parathyroidectomy. The donated kidney was from a deceased donor in 84% of the cases in both groups. Immunosuppression was similar in both groups. Table 1 shows the mean age, gender, type of immunosuppressant, duration of dialysis before transplantation, time of surgery after transplantation, mean preoperative creatinine and preoperative estimated glomerular filtration rate for patients in the two groups.
Demographic and clinical data.
| PTX Group (n = 19) | NON-PTX Group (n = 19) | P | |
|---|---|---|---|
| Age (years) | 44.7±10.7 | 48.5±12.3 | 0.20 |
| Sex Male (%) | 36% | 67% | 0.10 |
| IMMUNOSUPPRESSION Steroids | 100% | 100% | 1.0 |
| Cyclosporin A | 58% | 74% | 0.49 |
| Tacrolimus | 41% | 21% | 0.29 |
| Azathioprin | 21% | 32% | 0.71 |
| Mycophenolate mofetil or Sodium | 79% | 68% | 0.71 |
| Rapamycin | 0% | 5% | 1.0 |
| Duration of dialysis before transplantation (months) | 86.1 (0-204) | 57.2 (0-120) | 0.047 |
| Time after transplantation (months) | 36 (7-69) | 28 (3-76) | 0.23 |
| Creatinine before surgery (mg/dL) | 1.58 ± 0.6 | 1.49 ± 0.6 | 0.65 |
| Creatinine clearance (Cokcroft-Gault) (mL/minute) | 48.6 | 44.6 | 0.9 |
Legend: PTX = parathyroidectomy; NON-PTX = other operations.
In the parathyroidectomy group, the mean PTH was 350 pg/mL (range 134-3950) and the mean total calcium was 10.7 mg/dL (range 10.2-12.7). There were no operative deaths. A significant increase in serum creatinine from 1.58 to 2.29 mg/dL (p<0.05) was noted in the parathyroidectomy group within the first 5 days after parathyroidectomy. An insignificant increase in serum creatinine occurred in the control group in the first 5 days after surgery (baseline: 1.49mg/dL; day 5: 1.65mg/dL). On the fifth day after surgery, creatinine was higher in the parathyroidectomy group (p<0.05) than in the control group.
In the parathyroidectomy group, renal function stabilized after 24 ± 10 months of follow up. At that time, the mean serum creatinine value (1.91 mg/dL) was not statistically different from baseline. Renal function stabilized after 31 ± 24 months in the non-parathyroidectomy group, with a final follow-up creatinine mean value of 1.72 mg/dL (Figure 2).
At the long-term follow up, one death related to sepsis had occurred in the parathyroidectomy group, and two deaths related to cardiovascular disease had occurred in the non-parathyroidectomy group. None of the deaths were related to the surgical procedures. Censoring for death revealed similar findings. In the group of patients who underwent parathyroidectomy after the kidney transplantation, only two patients lost the graft, with one at 22 months post-surgery and the other at 63 months. The three patients were diagnosed with chronic allograft nephropathy. None of the controls lost their kidney grafts. The difference in graft loss was not statistically significant (p = 0.48).
DISCUSSIONThe decision to recommend parathyroid surgery after kidney transplantation is a difficult one. Evidence-based guidelines are currently lacking, and thus, laboratory data, clinical symptoms and imaging data should all be considered. A previous report suggested a possible deleterious effect of total parathyroidectomy with immediate autotransplantation on long-term kidney graft survival.8 Although the present study demonstrated an acute deterioration of renal function in the acute period after parathyroidectomy, renal function was similar to baseline and the control group at the final follow up. This suggests that the deterioration only persists short term. Group that contained patients undergoing some urological surgeries with a greater interference in renal function, the difference observed in the first week remained unchanged.
The present paper clearly shows that acute changes in renal function are not the same after parathyroidectomy and other comparable operations not involving the parathyroid glands, leading one to consider anesthetic or post-trauma effects on these variables.10 Indeed, one study demonstrated that renal function after total parathyroidectomy without immediate autotransplantation did not differ even from that of patients undergoing subtotal parathyroidectomy.11 This study emphasizes that persistent HPT is an important surgical disease in kidney transplant patients, as parathyroidectomy accounted for approximately 31% (28 of 90) of all operative diseases of these patients. Cinacalcet may play an effective role in the clinical management of such patients12, but the drug is not yet available for patients in Brazil.
The results presented here demonstrate that parathyroidectomy, which is an important therapeutic modality for kidney transplant patients, appears to be safe for kidney function irrespective of the technique employed. Notwithstanding, determining the mechanism involved in the acute deterioration of renal function that occurs shortly after parathyroidectomy may permit better patient selection and determination of the most appropriate type of parathyroidectomy in kidney-transplanted patients.13
Acute renal changes after parathyroidectomy are also seen in some patients undergoing parathyroidectomy for primary HPT. In the past, these alterations were clinically evidenced by the presence of oliguria, but it is now possible to observe a significant elevation in creatinine shortly after the surgery.14 Clinically evident renal failure may occur even in more recent cases.15,16
Much has been said in the literature about the hemodynamic effects of PTH on the kidney,17–19 but precise mechanisms for long-term alterations in renal function have not been proposed. Moreover, contradictory information has been presented.4 In 1983, Pang et al. suggested that different segments of the PTH molecule might have specific activities.20 It seems reasonable to speculate that the variable patterns of creatinine change after parathyroidectomy may result from the activity of another parathyroid substance or of a PTH fragment.
PTH-related protein (PTHrP) is an autocrine/paracrine peptide expressed in renal tubules and vasculature that may play an important role in regulating overall renal function. It was first identified as a tumor-derived protein responsible for humoral hypercalcemia of malignancy.21 PTHrP is homologous to PTH in the N-terminal region, suggesting a possible basis for the similarity of this peptide's actions to those of PTH when secreted into the systemic circulation by malignant tumors. Experimental analysis has shown that PTHrP can act as an endogenous vasorelaxant, modulating renal responses to vasoactive stimuli.16 This effect suggests the possibility that calcineurin inhibitors, which act as powerful glomerular vasoconstrictors, might worsen creatinine levels.22 In any case, both the acute and chronic effects of high levels of PTH on kidney function deserve further study, as apparently contradictory results have been described.23,24